Particle Size-Dependent Microstructure, Hardness and Electrochemical Corrosion Behavior of Atmospheric Plasma Sprayed NiCrBSi Coatings
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Characterizations
2.3. Electrochemical Measurements
3. Results and Discussion
3.1. Microstructural Features
3.2. Hardness
3.3. Electrochemical Results
3.4. Morphologies of the As-Sprayed Samples after Electrochemical Tests
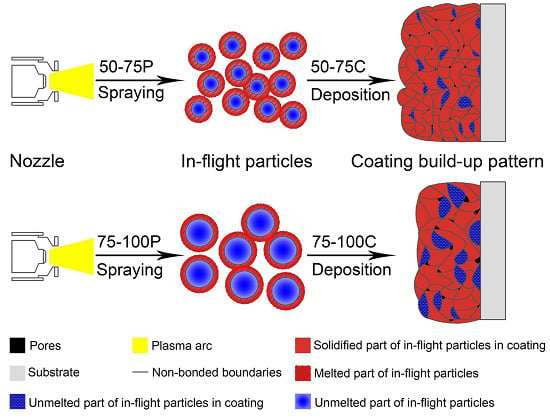
3.5. Mechanism for Particle Size Induced Distinctions
4. Conclusions
- (1)
- The use of powders with different sizes results in distinctive microstructures, including porosities, the volume fractions of amorphous phases and the number of non-bonded boundaries. The as-sprayed NiCrBSi coatings prepared from the powder with sizes of 50–75 μm (denoted as 50–75C) and 75–100 μm (denoted as 75–100C) exhibits different porosities (2.0 ± 0.8% for 50–75C and 3.0 ± 1.6% for 75–100C, respectively). The as-sprayed 50–75C has a lower hardness (700 ± 41 HV0.5) than the as-sprayed 75–100C (760 ± 74 HV0.5) in spite of the lower porosity. This is attributed to the larger volume fraction of the amorphous phase (15.5%) in the as-sprayed 50–75C compared with that of the counterpart (8.7%), which results from the higher melting degree of small in-flight particles. After eliminating the amorphous phase in the coatings by heat treatment, the porosity becomes the main factor that affects the hardness of the annealed NiCrBSi coatings. Hence, the annealed 50–75C shows a higher hardness (850 ± 111 HV0.5) than the annealed 75–100C (800 ± 82 HV0.5).
- (2)
- Due to the smaller powder used, a greater number of non-bonded boundaries are present in the as-sprayed 50–75C, leading to the easier penetration of corrosive media. Therefore, the as-sprayed 50–75C has higher corrosion current density (0.254 ± 0.062 μA/cm2) than the as-sprayed 75–100C (0.189 ± 0.069 μA/cm2). Correspondingly, the charge transfer resistance of the as-sprayed 50–75C (0.37 ± 0.07 MΩ cm2) is also lower than that of the as-sprayed 75–100C (0.50 ± 0.07 MΩ cm2).
- (3)
- The distinctions in the properties of both as-sprayed NiCrBSi coating samples mainly result from using different-sized powders. The same weight of powder would possess a larger total surface area due to the smaller size. Small in-flight particles are prone to be melted in the plasma arc and therefore the 50–75C show a larger volume fraction of the amorphous phase because of the fast solidification on the surface of the substrate. Meanwhile, owing to the limited diffusion between deposited particles, smaller particles also result in a greater number of non-bonded boundaries in the as-sprayed 50–75C, thereby degrading its corrosion resistance.
Author Contributions
Funding
Conflicts of Interest
References
- Lindner, T.; Löbel, M.; Lampke, T. Phase Stability and Microstructure Evolution of Solution-Hardened 316L Powder Feedstock for Thermal Spraying. Metals 2018, 8, 1063. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-Y.; Xu, T.; Wang, H.; Sang, P.; Lu, S.; Wang, Z.-X.; Chen, S.; Zhang, L.-C. Phase interaction induced texture in a plasma sprayed-remelted NiCrBSi coating during solidification: An electron backscatter diffraction study. Surf. Coat. Technol. 2019, 358, 467–480. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhang, Y.S.; Zhang, L.C. Transformation-induced plasticity and high strength in beta titanium alloy manufactured by selective laser melting. Materialia 2019, 6, 100299. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Liu, Y.; Li, S.; Hao, Y. Additive Manufacturing of Titanium Alloys by Electron Beam Melting: A Review. Adv. Eng. Mater. 2018, 20, 1700842. [Google Scholar] [CrossRef]
- Liu, Y.J.; Li, S.J.; Zhang, L.C.; Hao, Y.L.; Sercombe, T.B. Early plastic deformation behaviour and energy absorption in porous β-type biomedical titanium produced by selective laser melting. Scr. Mater. 2018, 153, 99–103. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Chen, L.-Y. A review on biomedical titanium alloys: Recent progress and prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.H.; Yang, C.; Kang, L.M.; Long, Y.; Xiao, Z.Y.; Li, P.J.; Zhang, L.C. Equiaxed Ti-based composites with high strength and large plasticity prepared by sintering and crystallizing amorphous powder. Mater. Sci. Eng. A 2016, 650, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Kang, L.M.; Li, X.X.; Zhang, W.W.; Zhang, D.T.; Fu, Z.Q.; Li, Y.Y.; Zhang, L.C.; Lavernia, E.J. Bimodal titanium alloys with ultrafine lamellar eutectic structure fabricated by semi-solid sintering. Acta Mater. 2017, 132, 491–502. [Google Scholar] [CrossRef]
- Yang, H.; Dong, E.; Zhang, B.; Yuan, Y.; Shu, S. Fabrication and Characterization of In Situ Synthesized SiC/Al Composites by Combustion Synthesis and Hot Press Consolidation Method. Scanning 2017, 2017, 9314740. [Google Scholar] [CrossRef]
- Qin, P.; Chen, Y.; Liu, Y.-J.; Zhang, J.; Chen, L.-Y.; Li, Y.; Zhang, X.; Cao, C.; Sun, H.; Zhang, L.-C. Resemblance in corrosion behavior of selective laser melted and traditional monolithic β Ti-24Nb-4Zr-8Sn alloy. ACS Biomater. Sci. Eng. 2019, 5, 1141–1149. [Google Scholar] [CrossRef]
- Chen, K.; Zeng, L.; Li, Z.; Chai, L.; Wang, Y.; Chen, L.-Y.; Yu, H. Effects of laser surface alloying with Cr on microstructure and hardness of commercial purity Zr. J. Alloys Compd. 2019, 784, 1106–1112. [Google Scholar] [CrossRef]
- Yang, Z.; Chu, C.; Jiang, F.; Qin, Y.; Long, X.; Wang, S.; Chen, D.; Zhang, F. Accelerating nano-bainite transformation based on a new constructed microstructural predicting model. Mater. Sci. Eng. A 2019, 748, 16–20. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Zhang, L.-C.; Chen, L.; Lu, W.; Zhang, D. Phase transformation and deformation behavior of NiTi-Nb eutectic joined NiTi wires. Sci. Rep. 2016, 6, 23905. [Google Scholar] [CrossRef] [PubMed]
- Leger, P.E.; Sennour, M.; Delloro, F.; Borit, F.; Debray, A.; Gaslain, F.; Jeandin, M.; Ducos, M. Multiscale Experimental and Numerical Approach to the Powder Particle Shape Effect on Al-Al2O3 Coating Build-Up. J. Therm. Spray Technol. 2017, 26, 1445–1460. [Google Scholar] [CrossRef]
- Wang, L.S.; Zhang, S.L.; Liu, T.; Li, C.J.; Li, C.X.; Yang, G.J. Dominant effect of particle size on the CeO2 preferential evaporation during plasma spraying of La2Ce2O7. J. Eur. Ceram. Soc. 2017, 37, 1577–1585. [Google Scholar] [CrossRef]
- Sun, Y.; Su, W.; Yang, H.; Ruan, J. Effects of WC particle size on sintering behavior and mechanical properties of coarse grained WC-8Co cemented carbides fabricated by unmilled composite powders. Ceram. Int. 2015, 41, 14482–14491. [Google Scholar] [CrossRef]
- Scendo, M.; Zorawski, W.; Goral, A. Influence of nickel powders on corrosion resistance of cold sprayed coatings on Al7075 substrate. Metals 2019, 9, 890. [Google Scholar] [CrossRef] [Green Version]
- Unabia, R.; Candidato, R.; Pawłowski, L. Current progress in solution precursor plasma spraying of cermets: A review. Metals 2018, 8, 420. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Lei, J.; Zhou, S.; Dai, X.; Zhang, L.C. Microstructure and mechanical properties of carbon fibers strengthened Ni-based coatings by laser cladding: The effect of carbon fiber contents. J. Alloys Compd. 2018, 744, 146–155. [Google Scholar] [CrossRef]
- Lei, J.; Shi, C.; Zhou, S.; Gu, Z.; Zhang, L.C. Enhanced corrosion and wear resistance properties of carbon fiber reinforced Ni-based composite coating by laser cladding. Surf. Coat. Technol. 2018, 334, 274–285. [Google Scholar] [CrossRef] [Green Version]
- Bobzin, K.; Öte, M.; Knoch, M.A.; Alkhasli, I. Macroscopic particle modeling in air plasma spraying. Surf. Coat. Technol. 2019, 364, 449–456. [Google Scholar] [CrossRef]
- Singh, H.; Sidhu, B.S.; Puri, D.; Prakash, S. Use of plasma spray technology for deposition of high temperature oxidation/corrosion resistant coatings—A review. Mater. Corros. 2007, 58, 92–102. [Google Scholar] [CrossRef]
- Li, C.-J.; Wang, W.-Z. Quantitative characterization of lamellar microstructure of plasma-sprayed ceramic coatings through visualization of void distribution. Mater. Sci. Eng. A 2004, 386, 10–19. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Wang, H.; Zhao, C.; Lu, S.; Wang, Z.-X.; Sha, J.; Chen, S.; Zhang, L.-C. Automatic remelting and enhanced mechanical performance of a plasma sprayed NiCrBSi coating. Surf. Coat. Technol. 2019, 369, 31–43. [Google Scholar] [CrossRef]
- Wei, Y.-K.; Li, Y.-J.; Zhang, Y.; Luo, X.-T.; Li, C.-J. Corrosion resistant nickel coating with strong adhesion on AZ31B magnesium alloy prepared by an in-situ shot-peening-assisted cold spray. Corros. Sci. 2018, 138, 105–115. [Google Scholar] [CrossRef]
- Chen, L.; Xu, T.; Lu, S.; Wang, Z.; Chen, S.; Zhang, L. Improved hardness and wear resistance of plasma sprayed nanostructured NiCrBSi coating via short-time heat treatment. Surf. Coat. Technol. 2018, 350, 436–444. [Google Scholar] [CrossRef]
- Li, C.-J.; Wang, Y.-Y.; Li, H. Effect of nano-crystallization of high velocity oxy-fuel-sprayed amorphous NiCrBSi alloy on properties of the coatings. J. Vac. Sci. Technol. A 2004, 22, 2000–2004. [Google Scholar] [CrossRef]
- Zhang, L.C.; Kim, K.B.; Yu, P.; Zhang, W.Y.; Kunz, U.; Eckert, J. Amorphization in mechanically alloyed (Ti, Zr, Nb)-(Cu, Ni)-Al equiatomic alloys. J. Alloys Compd. 2007, 428, 157–163. [Google Scholar] [CrossRef]
- Rabadia, C.D.; Liu, Y.J.; Wang, L.; Sun, H.; Zhang, L.C. Laves phase precipitation in Ti-Zr-Fe-Cr alloys with high strength and large plasticity. Mater. Des. 2018, 154, 228–238. [Google Scholar] [CrossRef]
- Zhang, L.C.; Shen, Z.Q.; Xu, J. Mechanically milling-induced amorphization in Sn-containing Ti-based multicomponent alloy systems. Mater. Sci. Eng. A 2005, 394, 204–209. [Google Scholar] [CrossRef]
- Houdková, Š.; Smazalová, E.; Vostřák, M.; Schubert, J. Properties of NiCrBSi coating, as sprayed and remelted by different technologies. Surf. Coat. Technol. 2014, 253, 14–26. [Google Scholar] [CrossRef]
- Yang, X.C.; Li, G.L.; Wang, H.D.; Dong, T.S.; Kang, J.J. Effect of flame remelting on microstructure and wear behaviour of plasma sprayed NiCrBSi-30%Mo coating. Surf. Eng. 2018, 34, 181–188. [Google Scholar] [CrossRef]
- Serres, N.; Hlawka, F.; Costil, S.; Langlade, C.; MacHi, F. Microstructures and mechanical properties of metallic NiCrBSi and composite NiCrBSi-WC layers manufactured via hybrid plasma/laser process. Appl. Surf. Sci. 2011, 257, 5132–5137. [Google Scholar] [CrossRef]
- Niranatlumpong, P.; Koiprasert, H. The effect of Mo content in plasma-sprayed Mo-NiCrBSi coating on the tribological behavior. Surf. Coat. Technol. 2010, 205, 483–489. [Google Scholar] [CrossRef]
- Deschuyteneer, D.; Petit, F.; Gonon, M.; Cambier, F. Processing and characterization of laser clad NiCrBSi/WC composite coatings—Influence of microstructure on hardness and wear. Surf. Coat. Technol. 2015, 283, 162–171. [Google Scholar] [CrossRef]
- Li, S.; Guo, Z.; Xiong, J.; Lei, Y.; Li, Y.; Tang, J.; Liu, J.; Ye, J. Corrosion behavior of HVOF sprayed hard face coatings in alkaline-sulfide solution. Appl. Surf. Sci. 2017, 416, 69–77. [Google Scholar] [CrossRef]
- Zhang, L.C.; Jia, Z.; Lyu, F.; Liang, S.X.; Lu, J. A review of catalytic performance of metallic glasses in wastewater treatment: Recent progress and prospects. Prog. Mater. Sci. 2019, 105, 100576. [Google Scholar] [CrossRef]
- Zhang, L.C.; Xu, J. Glass-forming ability of melt-spun multicomponent (Ti, Zr, Hf)–(Cu, Ni, Co)–Al alloys with equiatomic substitution. J. Non. Cryst. Solids 2004, 347, 166–172. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Xu, J.; Eckert, J. Thermal stability and crystallization kinetics of mechanically alloyed TiC/Ti-based metallic glass matrix composite. J. Appl. Phys. 2006, 100, 033514. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Zheng, X.; Geng, G. Dry sliding wear behavior and corrosion resistance of NiCrBSi coating deposited by activated combustion-high velocity air fuel spray process. Mater. Des. 2010, 31, 913–917. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, L.; Xu, H.; Xiao, J.; Zhang, G.; Liao, H. Role of Mo on tribological properties of atmospheric plasma-sprayed Mo-NiCrBSi composite coatings under dry and oil-lubricated conditions. J. Alloys Compd. 2017, 727, 841–850. [Google Scholar] [CrossRef]
- Dong, S.; Song, B.; Liao, H.; Coddet, C. Deposition of NiCrBSi coatings by atmospheric plasma spraying and dry-ice blasting: Microstructure and wear resistance. Surf. Coat. Technol. 2015, 268, 36–45. [Google Scholar] [CrossRef]
- Huang, S.; Sun, D.; Xu, D.; Wang, W.; Xu, H. Microstructures and Properties of NiCrBSi/WC Biomimetic Coatings Prepared by Plasma Spray Welding. J. Bionic Eng. 2015, 12, 592–603. [Google Scholar] [CrossRef]
- Bergant, Z.; Grum, J. Quality improvement of flame sprayed, heat treated, and remelted NiCrBSi coatings. J. Therm. Spray Technol. 2009, 18, 380–391. [Google Scholar] [CrossRef]
- Liu, L.; Xu, H.; Xiao, J.; Wei, X.; Zhang, G.; Zhang, C. Effect of heat treatment on structure and property evolutions of atmospheric plasma sprayed NiCrBSi coatings. Surf. Coat. Technol. 2017, 325, 548–554. [Google Scholar] [CrossRef]
- Zhang, L.C.; Xu, J.; Ma, E. Mechanically Alloyed Amorphous Ti50(Cu0.45Ni0.55)44–xAlxSi4B2 Alloys with Supercooled Liquid Region. J. Mater. Res. 2002, 17, 1743–1749. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.C.; Xu, J.; Ma, E. Consolidation and properties of ball-milled Ti50Cu18Ni22Al4Sn6 glassy alloy by equal channel angular extrusion. Mater. Sci. Eng. A 2006, 434, 280–288. [Google Scholar] [CrossRef]
- Calin, M.; Zhang, L.C.; Eckert, J. Tailoring of microstructure and mechanical properties of a Ti-based bulk metallic glass-forming alloy. Scr. Mater. 2007, 57, 1101–1104. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, L.C.; Zhang, W.Y.; Das, J.; Kim, K.B.; Eckert, J. Interfacial reaction during the fabrication of Ni60Nb40 metallic glass particles-reinforced Al based MMCs. Mater. Sci. Eng. A 2007, 444, 206–213. [Google Scholar] [CrossRef]
- Zhang, L.C.; Shen, Z.Q.; Xu, J. Glass formation in a (Ti, Zr, Hf)–(Cu, Ni, Ag)–Al high-order alloy system by mechanical alloying. J. Mater. Res. 2003, 18, 2141–2149. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.Y.; Shen, P.; Zhang, L.; Lu, S.; Chai, L.; Yang, Z.; Zhang, L.C. Corrosion behavior of non-equilibrium Zr-Sn-Nb-Fe-Cu-O alloys in high-temperature 0.01 M LiOH aqueous solution and degradation of the surface oxide films. Corros. Sci. 2018, 136, 221–230. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Wang, Z.; Shu, S.-L.; Lu, J.-B. Effect of Ta addition on the microstructures and mechanical properties of in situ bi-phase (TiB2-TiCxNy)/(Ni-Ta) cermets. Ceram. Int. 2019, 45, 4408–4417. [Google Scholar] [CrossRef]
- Lang, F.; Yu, Z. The corrosion resistance and wear resistance of thick TiN coatings deposited by arc ion plating. Surf. Coat. Technol. 2001, 145, 80–87. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.-Y.; Zhao, C.; Liu, Y.; Zhang, L.-C. Calculation of oxygen diffusion coefficients in oxide films formed on low-temperature annealed Zr alloys and their related corrosion behavior. Metals 2019, 9, 850. [Google Scholar] [CrossRef] [Green Version]
- Dai, N.; Zhang, L.-C.; Zhang, J.; Chen, Q.; Wu, M. Corrosion behavior of selective laser melted Ti-6Al-4V alloy in NaCl solution. Corros. Sci. 2016, 102, 484–489. [Google Scholar] [CrossRef]
- Xie, F.; He, X.; Cao, S.; Mei, M.; Qu, X. Influence of pore characteristics on microstructure, mechanical properties and corrosion resistance of selective laser sintered porous Ti-Mo alloys for biomedical applications. Electrochim. Acta 2013, 105, 121–129. [Google Scholar] [CrossRef]
- Bai, Y.; Gai, X.; Li, S.; Zhang, L.-C.; Liu, Y.; Hao, Y.; Zhang, X.; Yang, R.; Gao, Y. Improved corrosion behaviour of electron beam melted Ti–6Al–4V alloy in phosphate buffered saline. Corros. Sci. 2017, 123, 289–296. [Google Scholar] [CrossRef]
- Bandeira, R.M.; van Drunen, J.; Garcia, A.C.; Tremiliosi-Filho, G. Influence of the thickness and roughness of polyaniline coatings on corrosion protection of AA7075 aluminum alloy. Electrochim. Acta 2017, 240, 215–224. [Google Scholar] [CrossRef]
- Lu, H.B.; Zhang, L.C.; Gebert, A.; Schultz, L. Pitting corrosion of Cu—Zr metallic glasses in hydrochloric acid solutions. J. Alloys Compd. 2008, 462, 60–67. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Qin, X.; Guo, X.; Lu, J.; Chen, L.; Qin, J.; Lu, W. Erosion-wear and intergranular corrosion resistance properties of AISI 304L austenitic stainless steel after low-temperature plasma nitriding. J. Alloys Compd. 2017, 698, 1094–1101. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Dai, N.; Qin, P.; Attar, H.; Zhang, L.-C. Corrosion behaviour of selective laser melted Ti-TiB biocomposite in simulated body fluid. Electrochim. Acta 2017, 232, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Neville, A.; Hodgkiess, T. Electrochemical Study of the Localized Corrosion of Vacuum-Furnace-Fused Cermet Coatings. J. Am. Ceram. Soc. 2010, 82, 2133–2144. [Google Scholar] [CrossRef]
- Liang, S.X.; Jia, Z.; Liu, Y.J.; Zhang, W.; Wang, W.; Lu, J.; Zhang, L.C. Compelling Rejuvenated Catalytic Performance in Metallic Glasses. Adv. Mater. 2018, 30, 1802764. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.-C.; Liang, S.-X. Fe-based Metallic Glasses in Functional Catalytic Applications. Chem. Asian J. 2018, 13, 3575–3592. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Zhang, Y.; Lu, W.; Zhang, L.C.; Wang, L.; Zhang, D. Effect of low-temperature pre-deformation on precipitation behavior and microstructure of a Zr-Sn-Nb-Fe-Cu-O alloy during fabrication. J. Nucl. Sci. Technol. 2016, 53, 496–507. [Google Scholar] [CrossRef]
- Song, D.; Yang, J.; Yang, B.; Chen, L.; Wang, F.; Zhu, X. Evolution of structure and ferroelectricity in Aurivillius Bi4Bin-3Fen-3Ti3O 3n+3 thin films. J. Mater. Chem. C 2018, 6, 8618–8627. [Google Scholar] [CrossRef]
- Sadeghimeresht, E.; Reddy, L.; Hussain, T.; Huhtakangas, M.; Markocsan, N.; Joshi, S. Influence of KCl and HCl on high temperature corrosion of HVAF-sprayed NiCrAlY and NiCrMo coatings. Mater. Des. 2018, 148, 17–29. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, Q.; Li, J.; Lu, J.; Zhang, Y.; Zhang, L.-C.; Qin, X.; Lu, W.; Zhang, L.; Wang, L.; et al. Effect of microstructure on corrosion behavior of a Zr-Sn-Nb-Fe-Cu-O alloy. Mater. Des. 2016, 92, 888–896. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Sang, P.; Zhang, L.; Song, D.; Chu, Y.-Q.; Chai, L.; Zhang, L.-C. Homogenization and growth behavior of second-phase particles in a deformed Zr–Sn-Nb-Fe-Cu-Si-O alloy. Metals 2018, 8, 759. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.M.; Wang, Y.; Han, T.; Wu, K.Y.; Xue, J. Electrochemical evaluation of corrosion resistance of NiCrBSi coatings deposited by HVOF. Surf. Coat. Technol. 2004, 183, 118–125. [Google Scholar] [CrossRef]
- Mitsuhashi, A.; Asami, K.; Kawashima, A.; Hashimoto, K. The corrosion behavior of amorphous nickel base alloys in a hot concentrated phosphoric acid. Corros. Sci. 1987, 27, 957–970. [Google Scholar] [CrossRef]
















| Element | C | Si | B | Cr | Fe | Ni |
|---|---|---|---|---|---|---|
| Wt.% | 0.7~1.1 | 3.2~5.0 | 3.0~3.8 | 17.0~19.0 | <5.0 | Bal. |
| Spray Parameter | Measurement |
|---|---|
| Voltage (V) | 50 |
| Current (A) | 600 |
| Gun traverse rate (mm/s) | 100 |
| Main gas Ar (slpm) | 80 |
| Feed gas Ar (slpm) | 40 |
| Powder feed rate (g/s) | 0.3 |
| Spray distance (mm) | 80 |
| Sample | Ni | Ni3B | CrB | Cr3C2 | Cr7C3 | Amorphous Phase |
|---|---|---|---|---|---|---|
| As-sprayed 50–75C | 58.4 | 12.3 | 8.4 | 3.2 | 2.2 | 15.5 |
| As -sprayed 75C–100C | 63.3 | 13.5 | 8.9 | 3.5 | 2.1 | 8.7 |
| Annealed 50–75C | 66.9 | 18.4 | 8.8 | 3.5 | 2.4 | Undetected |
| Annealed 75C–100C | 67.2 | 18.7 | 8.2 | 3.6 | 2.3 | Undetected |
| Sample | Ecorr (mV) | Icorr (μA/cm2) |
|---|---|---|
| 50–75C | −332.7 | 0.243 |
| - | −325.3 | 0.184 |
| - | −298.1 | 0.334 |
| Average value | −318.7 | 0.254 |
| Std Dev | 14.9 | 0.062 |
| 75–100C | −315.8 | 0.134 |
| - | −316.9 | 0.149 |
| - | −304.2 | 0.286 |
| Average value | −312.3 | 0.189 |
| Std Dev | 5.7 | 0.069 |
| Sample | Rs (Ω cm2) | Rf (Ω cm2) | C × 10−6 (F) | Rct (MΩ cm2) | CPE × 10−6 (Ω−1 cm−2 S−n) | n | W × 10−5 (Ω−1 cm−2 S−0.5) | χ2 × 10−4 |
|---|---|---|---|---|---|---|---|---|
| 50–75C | 11.89 | 11.36 | 2.48 | 0.27 | 39.99 | 0.67 | 6.92 | 7.07 |
| 8.59 | 9.93 | 3.30 | 0.42 | 36.18 | 0.68 | 14.01 | 9.09 | |
| 11.79 | 4.21 | 0.95 | 0.41 | 32.56 | 0.66 | 12.77 | 4.39 | |
| Average value | 10.76 | 8.50 | 2.24 | 0.37 | 36.04 | 0.67 | 11.23 | 6.85 |
| Std Dev | 1.87 | 3.09 | 0.97 | 0.07 | 2.79 | 0.01 | 3.09 | - |
| 75–100C | 26.99 | 4.13 | 0.74 | 0.59 | 40.95 | 0.67 | 4.43 | 2.91 |
| 10.54 | 2.63 | 0.66 | 0.41 | 42.41 | 0.68 | 2.95 | 6.16 | |
| 13.08 | 3.65 | 0.72 | 0.49 | 37.38 | 0.67 | 2.38 | 9.57 | |
| Average value | 16.87 | 3.47 | 0.71 | 0.50 | 40.25 | 0.67 | 3.25 | 6.21 |
| Std Dev | 8.85 | 0.63 | 0.04 | 0.07 | 2.11 | 0.01 | 0.86 | - |
| Points | Location | Na (wt.%) | Cl (wt.%) | Ni (wt.%) | Si (wt.%) | Cr (wt.%) |
|---|---|---|---|---|---|---|
| A | at the interior of particles | 0.12 | 0.00 | 82.62 | 3.96 | 13.30 |
| B | at pores | 7.12 | 13.66 | 47.22 | 5.85 | 26.14 |
| C | at the interior of particles | 0.35 | 0.20 | 87.73 | 5.07 | 6.65 |
| D | at pores | 17.83 | 8.89 | 26.83 | 1.80 | 44.66 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sang, P.; Chen, L.-Y.; Zhao, C.; Wang, Z.-X.; Wang, H.; Lu, S.; Song, D.; Xu, J.-H.; Zhang, L.-C. Particle Size-Dependent Microstructure, Hardness and Electrochemical Corrosion Behavior of Atmospheric Plasma Sprayed NiCrBSi Coatings. Metals 2019, 9, 1342. https://doi.org/10.3390/met9121342
Sang P, Chen L-Y, Zhao C, Wang Z-X, Wang H, Lu S, Song D, Xu J-H, Zhang L-C. Particle Size-Dependent Microstructure, Hardness and Electrochemical Corrosion Behavior of Atmospheric Plasma Sprayed NiCrBSi Coatings. Metals. 2019; 9(12):1342. https://doi.org/10.3390/met9121342
Chicago/Turabian StyleSang, Peng, Liang-Yu Chen, Cuihua Zhao, Ze-Xin Wang, Haiyang Wang, Sheng Lu, Dongpo Song, Jia-Huan Xu, and Lai-Chang Zhang. 2019. "Particle Size-Dependent Microstructure, Hardness and Electrochemical Corrosion Behavior of Atmospheric Plasma Sprayed NiCrBSi Coatings" Metals 9, no. 12: 1342. https://doi.org/10.3390/met9121342