A Comprehensive Study of Steel Powders (316L, H13, P20 and 18Ni300) for Their Selective Laser Melting Additive Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and SLM Process
2.2. Characterization of Microstructure
3. Results
3.1. Phase Analysis of Powders
3.2. Cross-Sectional Microstructures of the Steel Powders
3.3. Surface Structures of the Steel Powders
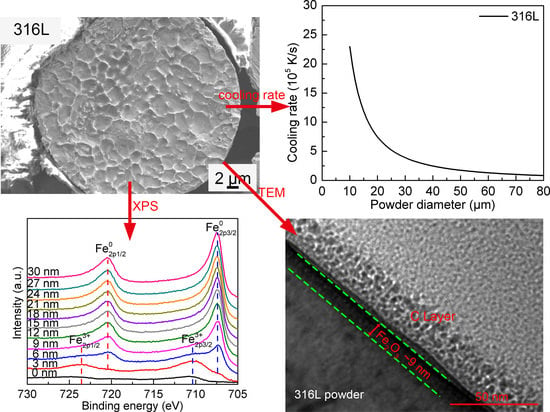
3.3.1. The 316L Steel Powder
3.3.2. The H13 Steel Powder
- Fe is present in the form of oxide at the surface of H13 and as the mixture of oxide and metal after etching up to 9 nm.
- Cr is present in oxide as Cr3+ from the outmost surface to the etching depth of 3 nm. From the depth of 6 nm to 15 nm, oxide Cr coexists with metallic Cr. Above the etch depth of 18 nm, only metallic chromium Cr0 is detectable.
- In the case of Mn, only manganese oxide is detected in the surface of H13, which is likely due to the high affinity of manganese to oxygen.
- For Mo, only metallic peaks are identified in the surface of the H13 powder.
3.3.3. The P20 Steel Powder
- The peaks of the Fe can be fitted into Fe3+ below the ion etching to the level of 3 nm and into metallic Fe after ion etching to the depth of 12 nm.
- For Cr, it can be deconvoluted into the Cr3+ from the outmost surface to etch depth of 6 nm, and into mixture of Cr3+ and Cr0 for 9 nm to 12 nm, and into metallic Cr0 only above etch depth of 15 nm.
- The chemical state of Mn in P20 remains as oxide.
- Mo in the P20 steel powder exists in the form of metallic state.
3.3.4. The 18Ni300 Steel Powder
- From the peaks of Fe, it can be found that the outmost surface is covered by oxide. Metallic Fe starts to appear at the etch depth of 3 nm, while metallic Fe0 is the majority at 6 nm etch depth. The peaks can only be fitted into metallic Fe0 when the etch depth is greater than 9 nm, representing fully removal of iron oxide.
- According to the characteristic of satellite peaks (at 858 eV), Ni is identified in the form of metallic state in the surface of the 18Ni300 powder.
3.4. Absorption Spectroscopy Results and SLM Processing Trials
4. Discussion
4.1. Relationship between Cooling Rate and Martensite Formation in the Steel Powders
4.2. Discussion on the Surface Structure of the Steel Powders
4.3. Effect of the Surface Oxide Layers on Laser Absorption
4.3.1. The Existence of Oxides Does Not Impede the Radiation of the Laser Beam on the Metal Matrix
4.3.2. The Existence of the Surface Oxide Layers Enhances the Laser Absorptivity of the Four Steel Powders
5. Conclusions
- (1)
- The phase of H13, P20 and 18Ni300 mainly consists of martensite and a small amount of austenite due to the high cooling rate (in the range of 105–106 K/s) during gas atomization processing, while 316L is composed of austenite phase.
- (2)
- Surfaces of these four steel powders are covered by heterogeneous oxides, composed of a thin continuous Fe2O3 layer with a thickness of about 9 nm and particulate particles of thermodynamically stable oxides (MnO, Cr2O3).
- (3)
- The measured absorptivity of these four steel powders is basically consistent with that calculated through the simplified model with considering the effect of oxide layer at the outer surface of powders.
- (4)
- The presence of the oxide layer on the powder surface does not impede the radiation of laser beam on the metal matrix. It can enhance the laser absorptivity of these four steel powders.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A



References
- Murr, L.E.; Gaytan, S.M.; Ramirez, D.A.; Martinez, E.; Hernandez, J.; Amato, K.N.; Shindo, P.W.; Medina, F.R.; Wicker, R.B. Metal Fabrication by Additive Manufacturing Using Laser and Electron Beam Melting Technologies. J. Mater. Sci. Technol. 2012, 28, 1–14. [Google Scholar] [CrossRef]
- Onal, E.; Frith, J.E.; Jurg, M.; Wu, X.H.; Molotnikov, A. Mechanical properties and in vitro behavior of additively manufactured and functionally graded Ti6Al4V porous scaffolds. Metals 2018, 8, 200. [Google Scholar] [CrossRef]
- Hebert, R.J. Viewpoint: Metallurgical aspects of powder bed metal additive manufacturing. J. Mater. Sci. 2016, 51, 1165–1175. [Google Scholar] [CrossRef]
- Luo, J.P.; Jia, X.; Gu, R.N.; Zhou, P.; Huang, Y.J.; Sun, J.F.; Yan, M. 316L stainless steel manufactured by selective laser melting and its biocompatibility with or without hydroxyapatite coating. Metals 2018, 8, 548. [Google Scholar] [CrossRef]
- Lu, Y.J.; Ren, L.; Wu, S.Q.; Yang, C.G.; Lin, W.L.; Xiao, S.L.; Yang, Y.; Yang, K.; Lin, J.X. CoCrWCu alloy with antibacterial activity fabricated by selective laser melting: Densification, mechanical properties and microstructural analysis. Powder Technol. 2018, 325, 289–300. [Google Scholar] [CrossRef]
- Brown, M.S.; Arnold, C.B. Fundamentals of laser-material interaction and application to multiscale surface modification. In Laser Precision Microfabrication; Sugioka, K., Meunier, M., Piqué, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 91–120. [Google Scholar]
- Heller, J.; Bartha, J.W.; Poon, C.C.; Tam, A.C. Temperature dependence of the reflectivity of silicon with surface oxide at wavelengths of 633 and 1047 nm. Appl. Phys. Lett. 1999, 75, 43–45. [Google Scholar] [CrossRef]
- Rubenchik, A.M.; Wu, S.S.Q.; Kanz, V.K.; LeBlanc, M.M.; Lowdermilk, W.H.; Rotter, M.D.; Stanley, J.R. Temperature-dependent 780-nm laser absorption by engineering grade aluminum, titanium, and steel alloy surfaces. Opt. Eng. 2014, 53, 122506:1–122506:8. [Google Scholar] [CrossRef]
- Mazur, M.; Brincat, P.; Leary, M.; Brandt, M. Numerical and experimental evaluation of a conformally cooled H13 steel injection mould manufactured with selective laser melting. Int. J. Adv. Manuf. Technol. 2017, 93, 881–900. [Google Scholar] [CrossRef]
- Yan, J.J.; Zheng, D.L.; Li, H.X.; Jia, X.; Sun, J.F.; Li, Y.L.; Qian, M.; Yan, M. Selective laser melting of H13: Microstructure and residual stress. J. Mater. Sci. 2017, 52, 12476–12485. [Google Scholar] [CrossRef]
- Casalino, G.; Campanelli, S.L.; Contuzzi, N.; Ludovico, A.D. Experimental investigation and statistical optimisation of the selective laser melting process of a maraging steel. Opt. Laser Technol. 2015, 65, 151–158. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Buciumeanu, M.; Pinto, E.; Alves, N.; Carvalho, O.; Silva, F.S.; Miranda, G. 316L stainless steel mechanical and tribological behavior—A comparison between selective laser melting, hot pressing and conventional casting. Addit. Manuf. 2017, 16, 81–89. [Google Scholar] [CrossRef]
- Kong, D.C.; Ni, X.Q.; Dong, C.F.; Lei, X.W.; Zhang, L.; Man, C.; Yao, J.Z.; Cheng, X.Q.; Li, X.G. Bio-functional and anti-corrosive 3D printing 316L stainless steel fabricated by selective laser melting. Mater. Des. 2018, 152, 88–101. [Google Scholar] [CrossRef]
- Buchanan, C.; Matilainen, V.P.; Salminen, A.; Gardner, L. Structural performance of additive manufactured metallic material and cross-sections. J. Constr. Steel Res. 2017, 136, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Capek, J.; Machova, M.; Fousova, M.; Kubásek, J.; Vojtěch, D.; Fojt, J.; Jablonská, E.; Lipov, J.; Ruml, T. Highly porous, low elastic modulus 316L stainless steel scaffold prepared by selective laser melting. Mater. Sci. Eng. C 2016, 69, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Rännar, L.E.; Wikman, S.; Koptyug, A.; Liu, L.F.; Cui, D.Q.; Shen, Z.J. Additive manufacturing of ITER first wall panel parts by two approaches: Selective laser melting and electron beam melting. Fusion Eng. Des. 2017, 116, 24–33. [Google Scholar] [CrossRef]
- Romei, F.; Grubišić, A.N.; Gibbon, D. Manufacturing of a high-temperature resistojet heat exchanger by selective laser melting. Acta Astronaut. 2017, 138, 356–368. [Google Scholar] [CrossRef] [Green Version]
- Jhavar, S.; Jain, N.K.; Paul, C.P. Development of micro-plasma transferred arc (μ-PTA) wire deposition process for additive layer manufacturing applications. J. Mater. Process. Technol. 2014, 214, 1102–1110. [Google Scholar] [CrossRef]
- Kempen, K.; Yasa, E.; Thijs, L.; Kruth, J.P.; Van Humbeeck, J. Microstructure and mechanical properties of Selective Laser Melted 18Ni-300 steel. Phys. Procedia 2011, 12, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Tolochko, N.K.; Khlopkov, Y.V.; Mozzharov, S.E.; Titov, V.I.; Ignatiev, M.B. Absorptance of powder materials suitable for laser sintering. Rapid Prototyp. J. 2000, 6, 155–161. [Google Scholar] [CrossRef]
- Bertoli, U.S.; Guss, G.; Wu, S.; Matthews, M.J.; Schoenung, J.M. In-situ characterization of laser-powder interaction and cooling rates through high-speed imaging of powder bed fusion additive manufacturing. Mater. Des. 2017, 135, 385–396. [Google Scholar] [CrossRef]
- Rubenchik, A.; Wu, S.; Mitchell, S.; Golosker, I.; LeBlanc, M.; Peterson, N. Direct measurements of temperature-dependent laser absorptivity of metal powders. Appl. Opt. 2015, 54, 7230–7233. [Google Scholar] [CrossRef] [PubMed]
- Boley, C.D.; Khairallah, S.A.; Rubenchik, A.M. Calculation of laser absorption by metal powders in additive manufacturing. Appl. Opt. 2015, 54, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.H.; Lin, S.F.; Hou, Y.H.; Wang, D.W.; Zhou, P.; Han, P.L.; Li, Y.L.; Yan, M. Layered surface structure of gas-atomized high Nb-containing TiAl powder and its impact on laser energy absorption for selective laser melting. Appl. Surf. Sci. 2018, 441, 210–217. [Google Scholar] [CrossRef]
- Trapp, J.; Rubenchik, A.M.; Guss, G.; Matthews, M.J. In situ absorptivity measurements of metallic powders during laser powder-bed fusion additive manufacturing. Appl. Mater. Today 2017, 9, 341–349. [Google Scholar] [CrossRef]
- Yang, T.Y.; Wen, W.; Yin, G.Z.; Li, X.L.; Gao, M.; Gu, Y.L.; Li, L.; Liu, Y.; Lin, H.; Zhang, X.M.; et al. Introduction of the X-ray diffraction beamline of SSRF. Nucl. Sci. Technol. 2015, 26, 1–5. [Google Scholar] [CrossRef]
- Gil, E.; Cortés, J.; Iturriza, I.; Ordás, N. XPS and SEM analysis of the surface of gas atomized powder precursor of ODS ferritic steels obtained through the STARS route. Appl. Surf. Sci. 2018, 427, 182–191. [Google Scholar] [CrossRef]
- Chasoglou, D.; Hryha, E.; Norell, M.; Nyborg, L. Characterization of surface oxides on water-atomized steel powder by XPS/AES depth profiling and nano-scale lateral surface analysis. Appl. Surf. Sci. 2013, 268, 496–506. [Google Scholar] [CrossRef]
- Hryha, E.; Gierl, C.; Nyborg, L.; Danninger, H.; Dudrova, E. Surface composition of the steel powders pre-alloyed with manganese. Appl. Surf. Sci. 2010, 256, 3946–3961. [Google Scholar] [CrossRef]
- Nyborg, L.; Nylund, A.; Olefjord, I. Thickness determination of oxide layers on spherically-shaped metallicpowders by ESCA. Surf. Interface Anal. 1988, 12, 110–114. [Google Scholar] [CrossRef]
- Irrinki, H.; Jangam, J.S.D.; Pasebani, S.; Badwe, S.; Stitzel, S.; Kate, K.; Gulsoy, O.; Atre, S.V. Effects of particle characteristics on the microstructure and mechanical properties of 17-4 PH stainless steel fabricated by laser-powder bed fusion. Powder Technol. 2018, 331, 192–203. [Google Scholar] [CrossRef]
- Yang, M.; Dai, Y.; Song, C.; Zhai, Q.J. Microstructure evolution of grey cast iron powder by high pressure gas atomization. J. Mater. Process. Technol. 2010, 210, 351–355. [Google Scholar] [CrossRef]
- Lee, E.S.; Ahn, S. Solification progress and heat transfer analysis of gas-atomized alloy droplets spray forming. Acta Metall. Mater. 1994, 42, 3231–3243. [Google Scholar] [CrossRef]
- Hussein, A.; Hao, L.; Yan, C.; Everson, R. Finite element simulation of the temperature and stress fields in single layers built without-support in selective laser melting. Mater. Des. 2013, 52, 638–647. [Google Scholar] [CrossRef]
- Foroozmehr, A.; Badrossamay, M.; Foroozmehr, E.; Golabi, S. Finite Element Simulation of Selective Laser Melting process considering Optical Penetration Depth of laser in powder bed. Mater. Des. 2016, 89, 255–263. [Google Scholar] [CrossRef]
- Wang, D.J. Thermal stability and sintering behavior of TiCuZrNiSn metallic glass. PhD Thesis, Harbin Institute of Technology, Harbin, China, 2010. [Google Scholar]
- Si, C.R.; Tang, X.L.; Zhang, X.J.; Wang, J.B.; Wu, W.C. Characteristics of 7055Al alloy powders manufactured by gas-solid two-phase atomization: A comparison with gas atomization process. Mater. Des. 2017, 118, 66–74. [Google Scholar] [CrossRef]
- Sun, Z.J.; Tan, X.P.; Tor, S.B.; Yeong, W.Y. Selective laser melting of stainless steel 316L with low porosity and high build rates. Mater. Des. 2016, 104, 197–204. [Google Scholar] [CrossRef]
- Krell, J.; Röttger, A.; Geenen, K.; Theisen, W. General investigations on processing tool steel X40CrMoV5-1 with selective laser melting. J. Mater. Process. Technol. 2018, 255, 679–688. [Google Scholar] [CrossRef]
- Dos Reis, A.G.; Reis, D.A.P.; Abdalla, A.J.; Otubo, J. High-temperature creep resistance and effects on the austenite reversion and precipitation of 18 Ni (300) maraging steel. Mater. Charact. 2015, 107, 350–357. [Google Scholar] [CrossRef]
- Oro, R.; Campos, M.; Hryha, E.; Torralba, J.M.; Nyborg, L. Surface phenomena during the early stages of sintering in steels modified with Fe–Mn–Si–C master alloys. Mater. Charact. 2013, 86, 80–91. [Google Scholar] [CrossRef]
- Simonelli, M.; Tuck, C.; Aboulkhair, N.T.; Maskery, I.; Ashcroft, I.; Wildman, R.D.; Hague, R. A Study on the Laser Spatter and the Oxidation Reactions During Selective Laser Melting of 316L Stainless Steel, Al-Si10-Mg, and Ti-6Al-4V. Metall. Mater. Trans. A 2015, 46A, 3842–3851. [Google Scholar] [CrossRef]
- Gaskell, D.R. Introduction to the Thermodynamics of Materials, 4th ed.; Taylor & Francis: New York, NY, USA, 2003. [Google Scholar]
- Smith, A.F. The tracer diffusion of transition metals in duplex oxide grown on a T316 stainless steel. Corros. Sci. 1981, 21, 517–529. [Google Scholar] [CrossRef]
- Smith, A.F. The Diffusion of Chromium in Type 316 Stainless Steel. Met. Sci. 2013, 9, 375–378. [Google Scholar] [CrossRef]
- Smitll, A.F.; Hales, R. Diffusion of Manganese in Type 316 Austenitic Stainless Steel. Met. Sci. 2013, 9, 181–184. [Google Scholar] [CrossRef]
- King, W.E.; Anderson, A.T.; Ferencz, R.M.; Hodge, N.E.; Kamath, C.; Khairallah, S.A.; Rubenchik, A.M. Laser powder bed fusion additive manufacturing of metals; physics, computational, and materials challenges. Appl. Phys. Rev. 2015, 2, 041304:1–041304:26. [Google Scholar] [CrossRef]
- Polyanskiy, M.N. Refractive Index Database. Available online: https://refractiveindex.info (accessed on 20 November 2018).
- Das, S. Physical aspects of process control in selective laser sintering of metals. Adv. Eng. Mater. 2003, 5, 701–711. [Google Scholar] [CrossRef]
- Yan, M.; Dargusch, M.S.; Ebel, T.; Qian, M. A transmission electron microscopy and three-dimensional atom probe study of the oxygen-induced fine microstructural features in as-sintered Ti–6Al–4V and their impacts on ductility. Acta Mater. 2014, 68, 196–206. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Zhang, Z.H.; Wang, Y.P.; Liu, G.; Zhou, S.Y.; Li, Y.L.; Shen, J.; Yan, M. Selective laser melting of typical metallic materials: An effective process prediction model developed by energy absorption and consumption analysis. Addit. Manuf. 2019, 25, 204–217. [Google Scholar] [CrossRef]











| Element | Cr | Ni | Mn | Mo | Si | Co | V | C | Fe | |
|---|---|---|---|---|---|---|---|---|---|---|
| Material | ||||||||||
| 316L | 16.5 | 10.5 | 1.8 | 2.25 | 0.5 | N/A | N/A | 0.03 | Bal. | |
| H13 | 4.88 | N/A | 0.45 | 1.22 | 1.1 | N/A | 1.03 | 0.36 | Bal. | |
| P20 | 1.95 | N/A | 0.83 | 0.33 | 0.45 | N/A | N/A | 0.40 | Bal. | |
| 18Ni300 | 0.3 | 18.2 | 0.04 | 5.0 | 0.1 | 9.0 | N/A | 0.01 | Bal. | |
| Element | Apparent Density (g/cm3) | Tapped Density (g/cm3) | Hausner Ratio (Tapped/Apparent) | |
|---|---|---|---|---|
| Material | ||||
| 316L | 4.27 | 5.12 | 1.20 | |
| H13 | 3.95 | 4.94 | 1.25 | |
| P20 | 4.21 | 5.03 | 1.19 | |
| 18Ni300 | 4.19 | 5.03 | 1.20 | |
| Power (W) | Scanning Speed (mm/s) | Layer Thickness (μm) | Hatching Space (μm) | Energy Density (J/mm3) | Relative Density | |
|---|---|---|---|---|---|---|
| 316L | 200 | 800 | 30 | 120 | 69.44 | 99.7% |
| H13 | 175 | 720 | 30 | 100 | 81.0 | 99.6% |
| P20 | 200 | 800 | 30 | 105 | 79.4 | 99.5% |
| 18Ni300 | 200 | 800 | 30 | 105 | 79.4 | 99.6% |
| Material | Measured Absorptivity | Calculated Absorptivity | Difference |
|---|---|---|---|
| Pure 316L | 0.36 | - | - |
| 316L powder + oxide | 0.55 | 0.57 | 3.64% |
| Pure H13 | 0.38 | - | - |
| H13 powder + oxide | 0.60 | 0.57 | 5% |
| Pure P20 | 0.37 | - | - |
| P20 powder + oxide | 0.56 | 0.57 | 1.79% |
| Pure 18Ni300 | 0.37 | - | - |
| 18Ni300 powder + oxide | 0.59 | 0.57 | 3.39% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Zhou, Y.; Gu, R.; Zhang, X.; Quach, W.-M.; Yan, M. A Comprehensive Study of Steel Powders (316L, H13, P20 and 18Ni300) for Their Selective Laser Melting Additive Manufacturing. Metals 2019, 9, 86. https://doi.org/10.3390/met9010086
Yan J, Zhou Y, Gu R, Zhang X, Quach W-M, Yan M. A Comprehensive Study of Steel Powders (316L, H13, P20 and 18Ni300) for Their Selective Laser Melting Additive Manufacturing. Metals. 2019; 9(1):86. https://doi.org/10.3390/met9010086
Chicago/Turabian StyleYan, Jujie, Yinghao Zhou, Ruinan Gu, Xingmin Zhang, Wai-Meng Quach, and Ming Yan. 2019. "A Comprehensive Study of Steel Powders (316L, H13, P20 and 18Ni300) for Their Selective Laser Melting Additive Manufacturing" Metals 9, no. 1: 86. https://doi.org/10.3390/met9010086