Leaching Behavior of Al, Co and W from the Al-Alloying Treated WC-Co Tool as a New Recycling Process for WC Hard Scrap
Abstract
:1. Introduction
2. Experimental
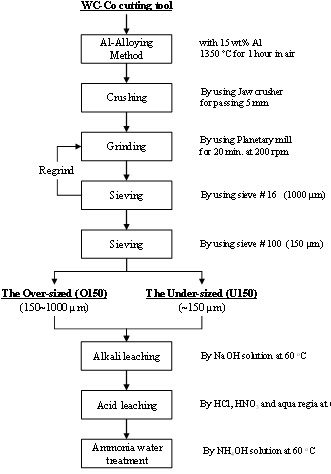
2.1. Preparation of the Starting Powder by Pulverization of a WC-Co Cutting Tool
2.2. Starting Materials and Their Characterization
3. Results and Discussion
3.1. Characterization of the Starting Powders of the Al-Alloyed WC-Co Cutting ToolS
3.2. Leaching Treatment for the Al-Alloyed WC-Co Powder
3.2.1. Alkali Leaching with NaOH
3.2.2. Acid Leaching
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Penrice, T.W. Alternative binders for hard metals. J. Mater. Shap. Technol. 1987, 5, 35–39. [Google Scholar] [CrossRef]
- Ogwu, A.A.; Davies, T.J. Proposed selection rules for suitable binders in cemented hard metals with possible applications for improving ductility in intermetallics. J. Mater. Sci. 1992, 27, 5382–5388. [Google Scholar] [CrossRef]
- Paul, R.L.; Te Riele, W.A.M.; Nicol, M.J. A novel process for recycling tungsten carbide scrap. Inter. J. Miner. Process. 1985, 15, 41–56. [Google Scholar] [CrossRef]
- Grzesik, W. Advanced Machining Processes of Metallic Materials: Theory, Modelling and Applications, 1st ed.; Elsevier: Oxford, UK, 2008. [Google Scholar]
- Prakash, L.J. Application of fine grained tungsten carbide based cemented carbides. Int. J. Refract. Met. Hard Mater. 1995, 13, 257–264. [Google Scholar] [CrossRef]
- Lee, D.J.; Yoo, C.S. Predicting a promising fusion technology in geoscience and mineral resources engineering using Korean patent data. Geosystem Eng. 2014, 17, 34–42. [Google Scholar] [CrossRef]
- Nakamura, T. E-scrap recycling system and technologies in Japan. Geosystem Eng. 2014, 17, 104–112. [Google Scholar] [CrossRef]
- Shibata, J.; Murayama, N.; Niinae, M. Recovery of tungsten and cobalt from tungsten carbide tool waste by hydrometallurgical method. Geosystem Eng. 2014, 17, 120–124. [Google Scholar] [CrossRef]
- Han, K.N.; Kellar, J.J.; Cross, W.M.; Safarzadeh, S. Opportunities and challenges for treating rare-earth elements. Geosystem Eng. 2014, 17, 178–194. [Google Scholar] [CrossRef]
- Kim, J.R.; Song, Y.E.; Munussami, G.; Kim, C.M.; Jeon, B.H. Recent applications of bioelectrochemical system for useful resource recovery: Retrieval of nutrient and metal from wastewater. Geosystem Eng. 2015, 18, 173–180. [Google Scholar] [CrossRef]
- Seyed Aizadeh Ganji, S.M.; Shafaei, S.Z.; Goudarzi, N.; Azizi, A. Investigating the best mixture extraction systems in the separation of rare earth elements from nitric acid solution using Cyanex272, D2EHPA, and 8-Hydroxyquinoline. Geosystem Eng. 2016, 19, 32–38. [Google Scholar] [CrossRef]
- Walraedt, J. The coldstream process: A new powder production equipment. Powder Metall. Int. 1970, 3, 24–27. [Google Scholar]
- Barnard, P.G.; Heine, K. Reclamation of refractory carbides from carbide materials. US Patent 3595484 A, 27 July 1971. [Google Scholar]
- Edward, M.T. Process of separating hard constituents from sintered hard metals. US Patent 2407752 A, 17 September 1946. [Google Scholar]
- Kojima, T.; Shimizu, T.; Sasai, R.; Itoh, H. Recycling process of WC-Co cermets by hydrothermal treatment. J. Mater. Sci. 2005, 40, 5167–5172. [Google Scholar] [CrossRef]
- Malyshev, V.V.; Gab, A.I. Resource-saving methods for recycling waste tungsten carbide-cobalt cermets and extraction of tungsten from tungsten concentrates. Theor. Found. Chem. Eng. 2007, 41, 436–441. [Google Scholar] [CrossRef]
- Ghandehari, M.H.; Faulkner, J.K.; Schussler, M. Selective Dissolution of the Binder Phase Alloy (Co-W) from WC-Co Cemented Carbides in Particulate Bed Electrode Systems. J. Electrochem. Soc. 1982, 129, 2666–2668. [Google Scholar] [CrossRef]
- Brookes, K.J.A. Reclaimed tungsten powders with ‘virgin’ properties. Met. Powder Rep. 1990, 45, 131–132. [Google Scholar] [CrossRef]
- Venkateswaran, S.; Schubert, W.D.; Lux, B.; Ostermann, M.; Kieffer, B. W-scrap recycling by the melt bath technique. Int. J. Refract. Met. Hard Mater. 1996, 14, 263–270. [Google Scholar] [CrossRef]
- Gu, W.H.; Jeong, Y.S.; Kim, K.M.; Kim, J.C.; Son, S.H.; Kim, S.J. Thermal oxidation behavior of WC–Co hard metal machining tool tip scraps. J. Mater. Process. Technol. 2012, 212, 1250–1256. [Google Scholar] [CrossRef]
- Kim, B.S.; Kwon, H.J.; No, K.M.; Seo, C.Y.; Choi, J.H. A method for crushing hard tungsten carbide scraps. Korea Patent 1015494130000, 27 August 2015. [Google Scholar]
- Kim, B.S.; Seo, C.Y.; No, K.M.; Choi, J.H.; Kwon, H.J. A method for crushing hard tungsten carbide scraps under Non-oxygen atmosphere. Korea Patent 1015725070000, 23 November 2015. [Google Scholar]






| Grade | UF10 | ISO range | K20-K50 |
|---|---|---|---|
| Composition | WC 90%, Co 10% | Grain size of WC | ~1 µm (submicron) |
| Density(g/cm3) | 14.45 | Shape | Square shape (length: 10 mm, thickness: 3 mm) |
| O150 | U150 | ||
|---|---|---|---|
| W | 72.00 | W | 46.71 |
| Co | 7.48 | Co | 6.33 |
| Al | 6.61 | Al | 19.03 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Lee, J.; Kim, B.; Kim, Y. Leaching Behavior of Al, Co and W from the Al-Alloying Treated WC-Co Tool as a New Recycling Process for WC Hard Scrap. Metals 2016, 6, 174. https://doi.org/10.3390/met6080174
Lee J, Lee J, Kim B, Kim Y. Leaching Behavior of Al, Co and W from the Al-Alloying Treated WC-Co Tool as a New Recycling Process for WC Hard Scrap. Metals. 2016; 6(8):174. https://doi.org/10.3390/met6080174
Chicago/Turabian StyleLee, Jaeryeong, Joowung Lee, Byoungjin Kim, and Youngjin Kim. 2016. "Leaching Behavior of Al, Co and W from the Al-Alloying Treated WC-Co Tool as a New Recycling Process for WC Hard Scrap" Metals 6, no. 8: 174. https://doi.org/10.3390/met6080174