Cooling-Rate Effect on Microstructure and Mechanical Properties of Al0.5CoCrFeNi High-Entropy Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Al0.5CoCrFeNi High-Entropy Alloy Powder
2.2. Al0.5CoCrFeNi High-Entropy Alloy Prepared by Spark Plasma Sintering
2.3. Heat Treatments
2.4. Microstructural and Mechanical Property Characterization
3. Results and Discussion
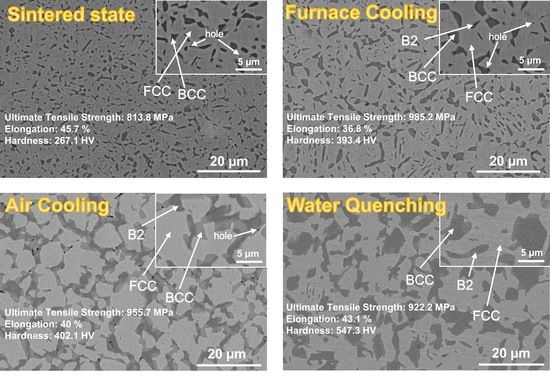
3.1. Microstructure
3.2. Mechanical Properties of Alloys
4. Conclusions
- The spark plasma sintered sample is composed of BCC + FCC phase, and the sample after heat treatment is composed of B2 + BCC + FCC phase. Heat treatment leads to the transformation of FCC to B2 + BCC phase and, with increasing cooling rate, further coarsening of BCC and B2 phases.
- The cooling rate has a significant effect on the tensile properties of the samples. The yield strength and ultimate tensile strength of the FC samples reached the highest values of 524.1 MP and 985.2 MPa, and the elongation was 36.8% (8.9% lower than that of the SS samples). The yield strength and ultimate tensile strength of the WQ samples are 477.5 MP and 922.2 MPa, respectively, and the elongation is 43.1% (only 2.6% lower than that of the sintered sample).
- The hardness of heat-treated samples is higher than that of SS samples, and the hardness value increases with the increase of cooling rate. The hardness of the WQ sample is 547.3 HV (280.2 HV higher than that of SS sample). The compactness of the samples after heat treatment is above 98.8%.
- Typical dimple features can be observed in the alloys after heat treatment, indicating that the alloys still have good plastic deformation ability, and their fracture mechanisms are typical plastic fractures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, Z.; Gao, M.C.; Diao, H.; Yang, T.; Liu, J.; Zuo, T.; Zhang, Y.; Lu, Z.; Cheng, Y.; Zhang, Y. Aluminum alloying effects on lattice types, microstructures, and mechanical behavior of high-entropy alloys systems. JOM 2013, 65, 1848–1858. [Google Scholar] [CrossRef]
- Rao, J.; Diao, H.; Ocelík, V.; Vainchtein, D.; Zhang, C.; Kuo, C.; Tang, Z.; Guo, W.; Poplawsky, J.; Zhou, Y. Secondary phases in alxcocrfeni high-entropy alloys: An in-situ tem heating study and thermodynamic appraisal. Acta Mater. 2017, 131, 206–220. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Senkov, O.N.; Parish, C.M.; Zhang, C.; Zhang, F.; Santodonato, L.J.; Wang, G.; Zhao, G.; Yang, F.; Liaw, P.K. Tensile ductility of an alcocrfeni multi-phase high-entropy alloy through hot isostatic pressing (hip) and homogenization. Mater. Sci. Eng. A 2015, 647, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Yang, B.; Xie, X.; Brechtl, J.; Dahmen, K.A.; Liaw, P.K. Corrosion of al x cocrfeni high-entropy alloys: Al-content and potential scan-rate dependent pitting behavior. Corros. Sci. 2017, 119, 33–45. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Qiao, J.W.; Ma, S.G.; Gao, M.C.; Yang, H.J.; Chen, M.W.; Zhang, Y. A hexagonal close-packed high-entropy alloy: The effect of entropy. Mater. Des. 2016, 96, 10–15. [Google Scholar] [CrossRef]
- Santodonato, L.J.; Zhang, Y.; Feygenson, M.; Parish, C.M.; Gao, M.C.; Weber, R.; Neuefeind, J.C.; Tang, Z.; Liaw, P.K. Deviation from high-entropy configurations in the atomic distributions of a multi-principal-element alloy. Nat. Commun. 2015, 6, 5964. [Google Scholar] [CrossRef] [Green Version]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. Cheminform abstract: A fracture-resistant high-entropy alloy for cryogenic applications. Sci. Technol. Weld. Join. 2014, 345, 1153. [Google Scholar] [CrossRef]
- Li, Z.; Tasan, C.C.; Pradeep, K.G.; Raabe, D. A trip-assisted dual-phase high-entropy alloy: Grain size and phase fraction effects on deformation behavior. Acta Mater. 2017, 131, 323–335. [Google Scholar] [CrossRef]
- Chuang, M.H.; Tsai, M.H.; Tsai, C.W.; Yang, N.H.; Chang, S.Y.; Yeh, J.W.; Chen, S.K.; Lin, S.J. Intrinsic surface hardening and precipitation kinetics of al0.3crfe1.5mnni0.5 multi-component alloy. J. Alloys Compd. 2013, 551, 12–18. [Google Scholar] [CrossRef]
- Li, R.; Niu, P.; Yuan, T.; Cao, P.; Chen, C.; Zhou, K. Selective laser melting of an equiatomic cocrfemnni high-entropy alloy: Processability, non-equilibrium microstructure and mechanical property. J. Alloys Compd. 2018, 746, 125–134. [Google Scholar] [CrossRef]
- Wu, W.; Song, M.; Ni, S.; Wang, J.; Liu, Y.; Liu, B.; Liao, X. Dual mechanisms of grain refinement in a fecocrni high-entropy alloy processed by high-pressure torsion. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, D.H.; Zhou, P.F.; Wu, W.Q.; Diao, H.Y.; Gao, M.C.; Song, M.; Liaw, P.K. Microstructure, mechanical and corrosion behaviors of alcocufeni-(cr,ti) high entropy alloys. Mater. Des. 2017, 116, 438–447. [Google Scholar] [CrossRef] [Green Version]
- Cantor, B.; Chang, I.; Knight, P.; Vincent, A. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yong, Z.A.; Ttz, A.; Zhi, T.B.; Mcgc, D.; Kad, E.; Pkl, B.; Zhao, P. Microstructures and properties of high-entropy alloys. Prog. Mater Sci. 2014, 61, 1–93. [Google Scholar]
- Butler, T.M.; Weaver, M.L. Investigation of the phase stabilities in alnicocrfe high entropy alloys. J. Alloys Compd. 2017, 691, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.S.; Kang, J.H.; Lim, K.R.; Na, Y.S. Influence of compressive strain on the microstructural evolution of an alcocrfeni high entropy alloy. Mater. Charact. 2017, 132, 162–168. [Google Scholar] [CrossRef]
- Wang, W.R.; Wang, W.L.; Yeh, J.W. Phases, microstructure and mechanical properties of alxcocrfeni high-entropy alloys at elevated temperatures. J. Alloys Compd. 2014, 589, 143–152. [Google Scholar] [CrossRef]
- Zhou, P.; Xiao, D.H.; Wu, Z.; Song, M. Microstructure and mechanical properties of alcocrfeni high entropy alloys produced by spark plasma sintering. Mater. Res. Express 2019, 6, 0865e7. [Google Scholar] [CrossRef]
- Wang, F.J.; Zhang, Y.; Chen, G.L.; Davies, H.A. Cooling rate and size effect on the microstructure and mechanical properties of alcocrfeni high entropy alloy. J. Eng. Mater. Technol. 2009, 131, 034501. [Google Scholar] [CrossRef]
- Butler, T.M.; Weaver, M.L. Oxidation behavior of arc melted alcocrfeni multi-component high-entropy alloys. J. Alloys Compd. 2016, 647, 229–244. [Google Scholar] [CrossRef]
- Karlsson, D.; Lindwall, G.; Lundbäck, A.; Amnebrink, M.; Boström, M.; Riekehr, L.; Schuisky, M.; Sahlberg, M.; Jansson, U. Binder jetting of the alcocrfeni alloy. Addit. Manuf. 2019, 27, 72–79. [Google Scholar] [CrossRef]
- Uporov, S.; Bykov, V.; Pryanichnikov, S.; Shubin, A.; Uporova, N. Effect of synthesis route on structure and properties of alcocrfeni high-entropy alloy. Intermetallics 2017, 83, 1–8. [Google Scholar] [CrossRef]
- Muthupandi, G.; Lim, K.R.; Na, Y.S.; Park, J.; Lee, D.; Kim, H.; Park, S.; Choi, Y.S. Pile-up and sink-in nanoindentation behaviors in alcocrfeni multi-phase high entropy alloy. Mater. Sci. Eng. A 2017, 696, 146–154. [Google Scholar] [CrossRef]
- Wu, Z.; Bei, H.; Otto, F.; Pharr, G.M.; George, E.P. Recovery, recrystallization, grain growth and phase stability of a family of fcc-structured multi-component equiatomic solid solution alloys. Intermetallics 2014, 46, 131–140. [Google Scholar] [CrossRef]
- Li, Y.; Lee, J.; Kang, B.; Hong, S.H. Microstructure and elevated-temperature mechanical properties of refractory almo0.5nbta0.5tizr high entropy alloy fabricated by powder metallurgy. arXiv 2017, arXiv:1801.00263. [Google Scholar]
- Ji, W.; Wang, W.; Wang, H.; Zhang, J.; Wang, Y.; Zhang, F.; Fu, Z. Alloying behavior and novel properties of cocrfenimn high-entropy alloy fabricated by mechanical alloying and spark plasma sintering. Intermetallics 2015, 56, 24–27. [Google Scholar] [CrossRef]
- Zhang, M.; Li, R.; Yuan, T.; Feng, X.; Xie, S. Effect of low-melting-point sintering aid on densification mechanisms of boron carbide during spark plasma sintering. Scripta Mater. 2019, 163, 34–39. [Google Scholar] [CrossRef]
- Deng, S.; Li, R.; Yuan, T.; Xie, S.; Zhang, M.; Zhou, K.; Cao, P. Direct current-enhanced densification kinetics during spark plasma sintering of tungsten powder. Scripta Mater. 2018, 143, 25–29. [Google Scholar] [CrossRef]
- Zhang, M.; Yuan, T.; Li, R.; Xie, S.; Wang, M.; Weng, Q. Densification mechanisms and microstructural evolution during spark plasma sintering of boron carbide powders. Ceram. Int. 2018, 44, 3571–3579. [Google Scholar] [CrossRef]
- Liu, G.; Li, R.; Yuan, T.; Zhang, M.; Zeng, F. Spark plasma sintering of pure ticn: Densification mechanism, grain growth and mechanical properties. Int. J. Refract. Met. Hard Mater. 2017, 66, 68–75. [Google Scholar] [CrossRef]
- Guillon, O.; Gonzalez-Julian, J.; Dargatz, B.; Kessel, T.; Schierning, G.; Räthel, J.; Herrmann, M. Field-assisted sintering technology/spark plasma sintering: Mechanisms, materials, and technology developments. Adv. Eng. Mater. 2014, 16, 830–849. [Google Scholar] [CrossRef]
- Giuntini, D.; Olevsky, E.A.; Garcia-Cardona, C.; Maximenko, A.L.; Yurlova, M.S.; Haines, C.D.; Martin, D.G.; Kapoor, D. Localized overheating phenomena and optimization of spark-plasma sintering tooling design. Materials 2013, 6, 2612–2632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, A.; Han, J.; Meng, J.; Su, B.; Li, P. Rapid preparation of alcocrfeni high entropy alloy by spark plasma sintering from elemental powder mixture. Mater. Lett. 2016, 181, 82–85. [Google Scholar] [CrossRef]
- Mohanty, S.; Maity, T.N.; Mukhopadhyay, S.; Sarkar, S.; Gurao, N.P. Powder metallurgical processing of equiatomic alcocrfeni high entropy alloy: Microstructure and mechanical properties. Mater. Sci. Eng. A 2017, 679, 299–313. [Google Scholar] [CrossRef]
- Shivam, V.; Shadangi, Y.; Basu, J.; Mukhopadhyay, N.K. Evolution of phases, hardness and magnetic properties of alcocrfeni high entropy alloy processed by mechanical alloying. J. Alloys Compd. 2020, 832, 154826. [Google Scholar] [CrossRef]
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-entropy alloys by mechanical alloying: A review. J. Mater. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Kunce, I.; Polanski, M.; Karczewski, K.; Plocinski, T.; Kurzydlowski, K.J. Microstructural characterisation of high-entropy alloy alcocrfeni fabricated by laser engineered net shaping. J. Alloys Compd. 2015, 648, 751–758. [Google Scholar] [CrossRef]
- Xie, S.; Li, R.; Yuan, T.; Zhang, M.; Cao, P. Effect of phase transformation on densification kinetics and properties of spark plasma sintered al0.7cocrfeni high-entropy alloy. Mater. Charact. 2019, 160, 110098. [Google Scholar] [CrossRef]
- Yang, T.; Xia, S.; Liu, S.; Wang, C.; Wang, Y. Effects of al addition on microstructure and mechanical properties of alxcocrfeni high-entropy alloy. Mater. Sci. Eng. A 2015, 648, 15–22. [Google Scholar] [CrossRef]
- Kattner, U.R. The thermodynamic modeling of multicomponent phase equilibria. JOM 1997, 49, 14–19. [Google Scholar] [CrossRef]
- Kube, S.A.; Schroers, J. Metastability in high entropy alloys. Scripta Mater. 2020, 186, 392–400. [Google Scholar] [CrossRef]
- Feng, Y.; Qiu, T. Preparation, characterization and microwave absorbing properties of feni alloy prepared by gas atomization method. J. Alloys Compd. 2012, 513, 455–459. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, B.; Yao, C. Comparative study of in600 superalloy produced by two powder metallurgy technologies: Argon atomizing and plasma rotating electrode process. Vacuum 2018, 156, 302–309. [Google Scholar]
- Gao, C.-f.; Xiao, Z.-y.; Zou, H.-p.; Liu, Z.-q.; Jin, C.; Li, S.-k.; Zhang, D.-t. Characterization of spherical alsi10mg powder produced by double-nozzle gas atomization using different parameters. Trans. Nonferrous Met. Soc. China 2019, 29, 374–384. [Google Scholar] [CrossRef]
- Munitz, A.; Salhov, S.; Hayun, S.; Frage, N. Heat treatment impacts the micro-structure and mechanical properties of alcocrfeni high entropy alloy. J. Alloys Compd. 2016, 683, 221–230. [Google Scholar] [CrossRef]
- Kao, Y.F.; Chen, T.J.; Chen, S.K.; Yeh, J.W. Microstructure and mechanical property of as-cast, -homogenized, and -deformed alxcocrfeni (0 <= x <= 2) high-entropy alloys. J. Alloys Compd. 2009, 488, 57–64. [Google Scholar] [CrossRef]
- Ji, W.; Fu, Z.; Wang, W.; Wang, H.; Zhang, J.; Wang, Y.; Zhang, F. Mechanical alloying synthesis and spark plasma sintering consolidation of cocrfenial high-entropy alloy. J. Alloys Compd. 2014, 589, 61–66. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Wang, J.; Niu, S.; Kou, H. Hot deformation behavior of as-cast and homogenized al0.5cocrfeni high entropy alloys. Metals 2016, 6, 277. [Google Scholar] [CrossRef] [Green Version]
- Ghaderi, A.; Moghanni, H.; Dehghani, K. Microstructural evolution and mechanical properties of al0.5cocrfeni high-entropy alloy after cold rolling and annealing treatments. J. Mater. Eng. Perform. 2021, 30, 7817–7825. [Google Scholar] [CrossRef]
- Zhou, P.F.; Xiao, D.H.; Yuan, T.C. Microstructure, mechanical and corrosion properties of alcocrfeni high-entropy alloy prepared by spark plasma sintering. Acta Metall. Sin. (Engl. Lett.) 2020, 33, 937–946. [Google Scholar] [CrossRef]
- Aizenshtein, M.; Priel, E.; Hayun, S. Effect of pre-deformation and b2 morphology on the mechanical properties of al0. 5cocrfeni hea. Mater. Sci. Eng. A 2020, 788, 139575. [Google Scholar] [CrossRef]
- Wrwa, B.; Wlw, B.; Scw, B.; Yct, B.; Chl, B.; Jwy, A. Effects of al addition on the microstructure and mechanical property of al x cocrfeni high-entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar]
- Lin, C.M.; Tsai, H.L. Evolution of microstructure, hardness, and corrosion properties of high-entropy al0.5cocrfeni alloy. Intermetallics 2011, 19, 288–294. [Google Scholar] [CrossRef]
- Lv, Y.; Hu, R.; Yao, Z.; Chen, J.; Xu, D.; Liu, Y.; Fan, X. Cooling rate effect on microstructure and mechanical properties of al x cocrfeni high entropy alloys. Mater. Des. 2017, 132, 392–399. [Google Scholar] [CrossRef]
- Wang, J.; Niu, S.; Guo, T.; Kou, H.; Li, J. The fcc to bcc phase transformation kinetics in an al 0.5 cocrfeni high entropy alloy. J. Alloys Compd. 2017, 710, 144–150. [Google Scholar] [CrossRef]
- Guo, T.; Li, J.; Wang, J.; Wang, W.Y.; Liu, Y.; Luo, X.; Kou, H.; Beaugnon, E. Microstructure and properties of bulk al 0.5 cocrfeni high-entropy alloy by cold rolling and subsequent annealing. Mater. Sci. Eng. A 2018, 729, 141–148. [Google Scholar] [CrossRef]
- Niu, S.Z.; Kou, H.C.; Wang, J.; Li, J.S. Improved tensile properties of al0.5cocrfeni high-entropy alloy by tailoring microstructures. Rare Met. 2021, 40, 1–6. [Google Scholar] [CrossRef]
- Tang, Q.H.; Zhao, Y.G.; Cai, J.B.; Dai, P.Q. Effect of aging treatment on microstructures and mechanical properties of al0.5cocrfeni high-entropy alloy. Trans. Nonferrous Met. Soc. China 2011, 4, 47–50. [Google Scholar]
- Xu, D.; Chen, K.; Chen, Y.; Chen, S. Evolution of the second-phase particles and their effect on tensile fracture behavior of 2219 al-xcu alloys. Metals 2020, 10, 197. [Google Scholar] [CrossRef] [Green Version]






| Al0.5CoCrFeNi | Phase | Chemical Composition/at.% | ||||
|---|---|---|---|---|---|---|
| Al | Co | Cr | Fe | Ni | ||
| Sintered state | FCC | 9.39 | 24.01 | 20.73 | 23.77 | 22.09 |
| BCC | 12.56 | 12.77 | 43.20 | 13.04 | 18.43 | |
| Furnace cooling | FCC | 6.92 | 22.90 | 20.19 | 26.08 | 23.91 |
| BCC | 2.72 | 11.72 | 61.98 | 17.50 | 6.08 | |
| B2 | 29.12 | 16.38 | 9.98 | 12.01 | 32.51 | |
| Air cooling | FCC | 9.26 | 26.52 | 13.17 | 26.94 | 24.11 |
| BCC | 2.66 | 5.63 | 78.71 | 9.38 | 3.63 | |
| B2 | 28.12 | 19.28 | 5.12 | 15.31 | 32.16 | |
| Water quenching | FCC | 10.03 | 29.90 | 5.42 | 28.94 | 25.71 |
| BCC | 2.42 | 13.93 | 54.39 | 24.23 | 5.03 | |
| B2 | 31.27 | 5.31 | 10.41 | 16.41 | 36.60 | |
| Al0.5CoCrFeNi | Yield Strength (MPa) | Ultimate Tensile Strength (MPa) | Elongation (%) | Hardness (HV) | Density (%) |
|---|---|---|---|---|---|
| Sintered state | 439.5 ± 3 | 813.8 ± 9 | 45.7 ± 0.6 | 267.1 ± 3 | 99.1 ± 0.6 |
| Furnace cooling | 524.1 ± 5 | 985.2 ± 6 | 36.8 ± 0.8 | 393.4 ± 5 | 99.2 ± 0.8 |
| Air cooling | 507.4 ± 3 | 955.7 ± 8 | 40.0 ± 1 | 402.1 ± 9 | 98.8 ± 1 |
| Water quenched | 477.5 ± 2 | 922.2 ± 4 | 43.1 ± 0.5 | 547.3 ± 8 | 99.3 ± 0.5 |
| Armin et al. [50] | 319 ± 10 | 468 ± 11 | 30 | 230 ± 12 | - |
| Jun et al. [56] | 707 | 1143 | 21.5 | - | - |
| Tong et al. [57] | 403 | 762 | 37.79 | - | - |
| Niu et al. [58] | 360 | 720 | 33 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, K.; Huang, L.; Wang, X.; Yu, L.; Feng, W. Cooling-Rate Effect on Microstructure and Mechanical Properties of Al0.5CoCrFeNi High-Entropy Alloy. Metals 2022, 12, 1254. https://doi.org/10.3390/met12081254
Xiong K, Huang L, Wang X, Yu L, Feng W. Cooling-Rate Effect on Microstructure and Mechanical Properties of Al0.5CoCrFeNi High-Entropy Alloy. Metals. 2022; 12(8):1254. https://doi.org/10.3390/met12081254
Chicago/Turabian StyleXiong, Ke, Lin Huang, Xiaofeng Wang, Lin Yu, and Wei Feng. 2022. "Cooling-Rate Effect on Microstructure and Mechanical Properties of Al0.5CoCrFeNi High-Entropy Alloy" Metals 12, no. 8: 1254. https://doi.org/10.3390/met12081254