Characterization of Defined Pt Particles Prepared by Ultrasonic Spray Pyrolysis for One-Step Synthesis of Supported ORR Composite Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.2. Material Characterization
2.2.1. Composition, Morphology, and Structural Characterization
2.2.2. Electrochemical Characterization
3. Results
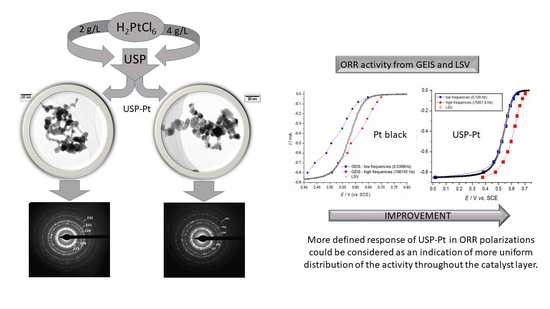
3.1. DLS, STEM, and TGA Characterization of Pt Samples
3.2. Electrochemical Properties of Obtained Pt Particles
3.2.1. Cyclic Voltammetry
3.2.2. Linear Sweep Voltammetry
3.2.3. Galvanostatic Electrochemical Impedance Spectroscopy (GEIS)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pei, Y.; Hu, M.; Xia, Y.; Huang, W.; Li, Z.; Chen, S. Electrochemical preparation of Pt nanoparticles modified nanoporous gold electrode with highly rough surface for efficient determination of hydrazine. Sens. Actuators B Chem. 2020, 304, 127416. [Google Scholar] [CrossRef]
- Mello, R.L.S. Preparation and electrochemical characterization of Pt nanoparticles dispersed on niobium oxide. Eclet. Quím. 2003, 28, 69–76. [Google Scholar] [CrossRef]
- Yasin, G.; Ibrahim, S.; Ibraheem, S.; Ali, S.; Iqbal, R.; Kumar, A.; Tabish, M.; Slimani, Y.; Nguyen, T.A.; Xu, H.; et al. Defective/graphitic synergy in a heteroatom-interlinked-triggered metal-free electrocatalyst for high-performance rechargeable zinc–air batteries. J. Mater. Chem. A 2021, 9, 18222. [Google Scholar] [CrossRef]
- Ibraheem, S.; Chen, S.; Peng, L.; Li, J.; Li, L.; Liao, Q.; Shao, M.; Wei, Z. Strongly coupled iron selenides-nitrogen-bond as an electronic transport bridge for enhanced synergistic oxygen electrocatalysis in rechargeable zinc-O2 batteries. Appl. Catal. B Environ. 2020, 265, 118569. [Google Scholar] [CrossRef]
- Ibraheem, S.; Chen, S.; Li, J.; Li, W.; Gao, X.; Wang, Q.; Wei, Z. Three-dimensional Fe,N-decorated carbon-supported NiFeP nanoparticles as an efficient bifunctional catalyst for rechargeable zinc−O2 batteries. ACS Appl. Mater. Inter. 2019, 11, 699–705. [Google Scholar] [CrossRef]
- Olabi, A.G.; Sayed, E.T.; Wilberforce, T.; Jamal, A.; Alami, A.H.; Elsaid, K.; Rahman, S.M.A.; Shah, S.K.; Abdelkareem, M.A. Metal-Air Batteries—A Review. Energies 2021, 14, 7373. [Google Scholar] [CrossRef]
- Ibraheem, S.; Yasin, G.; Kumar, A.; Mushtaq, M.A.; Ibrahim, S.; Iqbal, R.; Tabish, M.; Ali, S.; Saad, A. Iron-cation-coordinated cobalt-bridged-selenides nanorods for highly efficient photo/electrochemical water splitting. Appl. Catal. B Environ. 2022, 304, 120987. [Google Scholar] [CrossRef]
- Yasin, G.; Ibraheem, S.; Ali, S.; Arif, M.; Ibrahim, S.; Iqbal, R.; Kumar, A.; Tabish, M.; Mushtaq, M.A.; Saad, A.; et al. Defects-engineered tailoring of tri-doped interlinked metal-free bifunctional catalyst with lower gibbs free energy of OER/HER intermediates for overall water splitting. Mater. Today Chem. 2022, 23, 100634. [Google Scholar] [CrossRef]
- Nadeem, M.; Yasin, G.; Arif, M.; Tabassum, H.; Bhatti, M.H.; Mehmood, M.; Yunus, U.; Iqbal, R.; Nguyen, T.A.; Slimani, Y.; et al. Highly active sites of Pt/Er dispersed N-doped hierarchical porous carbon for trifunctional electrocatalyst. Chem. Eng. J. 2021, 409, 128205. [Google Scholar] [CrossRef]
- Motsoeneng, R.G.; Modibedi, R.M.; Mathe, M.K.; Khotseng, L.E.; Ozoemena, K.I. The synthesis of PdPt/carbon paper via surface limited redox replacement reactions for oxygen reduction reaction. Int. J. Hydrog. 2015, 40, 16734–16744. [Google Scholar] [CrossRef]
- Vidal-Iglesias, F.J.; Ara, R.M.; Solla-Gullo, J.; Herrero, E.; Feliu, J.M. Electrochemical Characterization of Shape-Controlled Pt Nanoparticles in Different Supporting Electrolytes. ACS Catal. 2012, 2, 901–910. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Lim, B.; Jang, H.-S.; Hwang, S.J.; Yoo, S.J.; Ha, J.S.; Cho, E.A.; Lim, T.-H.; Nam, S.W.; Kim, S.-K. Size-controlled synthesis of Pt nanoparticles and their electrochemical activities toward oxygen reduction. Int. J. Hydrog. Energy 2010, 36, 706–712. [Google Scholar] [CrossRef]
- Stepanov, A.L.; Golubev, A.N.; Nikitin, S.I.; Osin, Y.N. A review on the fabrication and properties of platinum nanoparticles. Rev. Adv. Mater. Sci. 2014, 38, 160–175. [Google Scholar]
- Tao, A.R.; Habas, S.; Yang, P. Shape control of colloidal metal nanocrystals. Small 2008, 4, 310–325. [Google Scholar] [CrossRef]
- Lau, M.; Gökce, B.; Marzun, G.; Rehbock, C.; Barcikowski, S. Rapid nanointegration with laser-generated nanoparticles. Lasers Manuf. Conf. 2015, 109, 1–7. [Google Scholar]
- Alkan, G.; Diaz, F.; Matula, G.; Stopic, S.; Friedrich, B. Scaling up of nanopowder collection in the process of ultrasonic spray pyrolysis. World Metall Erzmetall. 2017, 70, 97–101. [Google Scholar]
- Alkan, G.; Rudolf, R.; Bogovic, J.; Jenko, D.; Friedrich, B. Structure and Formation Model of Ag/TiO2 and Au/TiO2 Nanoparticles Synthesized through Ultrasonic Spray Pyrolysis. Metals 2017, 7, 389. [Google Scholar] [CrossRef] [Green Version]
- Messing, G.L.; Zhang, S.C.; Jayanthi, G.V. Ceramic Powder Synthesis by Spray Pyrolysis. J. Am. Ceram. Soc. 1993, 76, 2707–2726. [Google Scholar] [CrossRef]
- Gurav, A.; Kodas, T.; Pluym, T.; Xiong, Y. Aerosol processing of materials. Aerosol Sci. Technol. 1993, 19, 411–452. [Google Scholar] [CrossRef]
- Jung, C.H.; Yun, J.; Qadir, K.; Naik, B.; Yun, J.Y.; Park, J.Y. Catalytic activity of Pt/SiO2 nanocatalysts synthesized via ultrasonic spray pyrolysis process under CO oxidation. Appl. Catal. B Environ. 2014, 154–155, 171–176. [Google Scholar] [CrossRef]
- Jung, C.H.; Yun, J.; Qadir, K.; Park, D.; Yun, J.Y.; Park, J.Y. Pt/oxide nanocatalysts synthesized via the ultrasonic spray pyrolysis process: Engineering metal-oxide interfaces for enhanced catalytic activity. Res. Chem. Intermed. 2016, 42, 211–222. [Google Scholar] [CrossRef]
- Muñoz-Fernandez, L.; Alkan, G.; Milošević, O.; Rabanal, M.E.; Friedrich, B. Synthesis and characterisation of spherical core-shell Ag/ZnO nanocomposites using single and two–steps ultrasonic spray pyrolysis (USP). Catal. Today 2019, 321–322, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Rowston, W.B.; Ottaway, J.M. Determination of noble metals by carbon furnace atomic-absorption spectrometry. Part 1. Atom formation processes. Analyst 1979, 104, 645–659. [Google Scholar] [CrossRef]
- Košević, M.; Zarić, M.; Stopić, S.; Stevanovic, J.; Weirich, T.; Friedrich, B.; Panic, V. Structural and Electrochemical Properties of Nesting and Core/Shell Pt/TiO2 Spherical Particles Synthesized by Ultrasonic Spray Pyrolysis. Metals 2020, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Schweizer, A.E.; Kerr, G.T. Thermal Decomposition of Hexachloroplatinic Acid. Inorg. Chem. 1978, 17, 2326–2327. [Google Scholar] [CrossRef]
- Alkan, G.; Rudolf, R.; Emil, E.; Jenko, D.; Friedrich, B.; Gurmen, S. Tuning the Morphology of ZnO Nanostructures with the Ultrasonic Spray Pyrolysis Process. Metals 2018, 8, 569. [Google Scholar]
- Gharibshahi, E.; Saion, E. Influence of dose on particle size and optical properties of colloidal platinum nanoparticles. Int. J. Mol. Sci. 2012, 13, 14723–14741. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.B.; Nguyen, T.D.; Nguyen, Q.D.; Nguyen, T.T. Preparation of platinum nanoparticles in liquids by laser ablation method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 035011. [Google Scholar] [CrossRef] [Green Version]
- Du, C.; Sun, Y.; Shen, T.; Yin, G.; Zhang, J. 7 Applications of RDE and RRDE Methods in Oxygen Reduction Reaction. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts, 1st ed.; Xing, W., Yin, G., Zhang, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 231–277. [Google Scholar]
- Zorko, M.; Martins, P.F.B.D.; Connell, J.G.; Lopes, P.P.; Markovic, N.M.; Stamenkovic, V.R.; Strmcnik, D. Improved Rate for the Oxygen Reduction Reaction in a Sulfuric Acid Electrolyte using a Pt(111) Surface Modified with Melamine. ACS Appl. Mater. Interfaces 2021, 13, 3369–3376. [Google Scholar] [CrossRef]
- Devivaraprasad, R.; Ramesh, R.; Naresh, N.; Kar, T.; Singh, R.K.; Neergat, M. Oxygen reduction reaction and peroxide generation on shape-controlled and polycrystalline platinum nanoparticles in acidic and alkaline electrolytes. Langmuir 2014, 30, 8995–9006. [Google Scholar] [CrossRef]
- Grgur, B.; Marković, N.M.; Ross, P.N. Temperature dependent oxygen electrochemistry on platinum low index single crystal surfaces in acid solutions. Can. J. Chem. 1997, 75, 1465–1471. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkan, G.; Košević, M.; Mihailović, M.; Stopic, S.; Friedrich, B.; Stevanović, J.; Panić, V. Characterization of Defined Pt Particles Prepared by Ultrasonic Spray Pyrolysis for One-Step Synthesis of Supported ORR Composite Catalysts. Metals 2022, 12, 290. https://doi.org/10.3390/met12020290
Alkan G, Košević M, Mihailović M, Stopic S, Friedrich B, Stevanović J, Panić V. Characterization of Defined Pt Particles Prepared by Ultrasonic Spray Pyrolysis for One-Step Synthesis of Supported ORR Composite Catalysts. Metals. 2022; 12(2):290. https://doi.org/10.3390/met12020290
Chicago/Turabian StyleAlkan, Gözde, Milica Košević, Marija Mihailović, Srecko Stopic, Bernd Friedrich, Jasmina Stevanović, and Vladimir Panić. 2022. "Characterization of Defined Pt Particles Prepared by Ultrasonic Spray Pyrolysis for One-Step Synthesis of Supported ORR Composite Catalysts" Metals 12, no. 2: 290. https://doi.org/10.3390/met12020290