Adsorption Mechanism of Eco-Friendly Corrosion Inhibitors for Exceptional Corrosion Protection of Carbon Steel: Electrochemical and First-Principles DFT Evaluations
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Synthesis of Inhibitor Molecules
2.2. Samples and Corrosive Medium
2.3. Gravimetric Method
2.4. Assessment of Electrochemical Behavior Using LPR, EIS, and PDP
2.5. Computational Methods
2.6. Morphological Analysis
3. Results and Discussion
3.1. Long-Term Immersion by Weight Loss Study
3.2. PDP Measurements
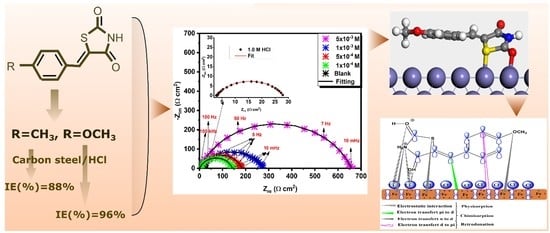
3.3. Electrochemical Behavior by EIS and LPR Assessment
3.4. Adsorption Isotherm Model
3.5. Morphological Characterization by SEM
3.6. First-Principles DFT Evaluations
3.6.1. Adsorption Configuration and Interaction Energy
3.6.2. Projected Density of States (PDOS)
3.7. Adsorption Mechanism of Adsorbed Molecules
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aboelnga, M.M.; Awad, M.K.; Gauld, J.W.; Mustafa, M.R. An assessment to evaluate the validity of different methods for the description of some corrosion inhibitors. J. Mol. Model. 2014, 20, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sanaei, Z.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Use of Rosa canina fruit extract as a green corrosion inhibitor for mild steel in 1M HCl solution: A complementary experimental, molecular dynamics and quantum mechanics investigation. J. Ind. Eng. Chem. 2019, 69, 18–31. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Bahlakeh, G.; Sanaei, Z.; Ramezanzadeh, B. Corrosion inhibition of mild steel in 1 M HCl solution by ethanolic extract of eco-friendly Mangifera indica (mango) leaves: Electrochemical, molecular dynamics, Monte Carlo and ab initio study. Appl. Surf. Sci. 2019, 463, 1058–1077. [Google Scholar] [CrossRef]
- Nikoo, S.Z.; Shockravi, A.; Ghartavol, H.M.; Halimehjani, A.Z.; Ostadrahimi, M.; Mirhosseini, S.M.; Behzadi, H.; Ghorbani, M. A study of glycine-based dithiocarbamates as effective corrosion inhibitors for cold rolled carbon steel in HCl solutions. Surf. Interfaces 2020, 21, 100751. [Google Scholar] [CrossRef]
- Tiwari, N.; Mitra, R.K.; Yadav, M. Corrosion protection of petroleum oil well/tubing steel using thiadiazolines as efficient corrosion inhibitor: Experimental and theoretical investigation. Surf. Interfaces 2021, 22, 100770. [Google Scholar] [CrossRef]
- Jafarpour, H.; Aghaei, H.; Litvin, V.; Ashena, R. Experimental optimization of a recently developed matrix acid stimulation technology in heterogeneous carbonate reservoirs. J. Pet. Sci. Eng. 2021, 196, 108100. [Google Scholar] [CrossRef]
- Solomon, M.M.; Umoren, S.A.; Quraishi, M.A.; Tripathy, D.B.; Abai, E.J. Effect of akyl chain length, flow, and temperature on the corrosion inhibition of carbon steel in a simulated acidizing environment by an imidazoline-based inhibitor. J. Pet. Sci. Eng. 2020, 187, 106801. [Google Scholar] [CrossRef]
- Hajjaji, F.E.; Salim, R.; Taleb, M.; Benhiba, F.; Rezki, N.; Chauhan, D.S.; Quraishi, M.A. Pyridinium-based ionic liquids as novel eco-friendly corrosion inhibitors for mild steel in molar hydrochloric acid: Experimental & computational approach. Surf. Interfaces 2021, 22, 100881. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Faro, L.V.; Pina, V.G.S.S.; de Souza, M.C.B.V.; Boechat, F.C.S.; de Souza, M.C.; Briganti, M.; Totti, F.; Ponzio, E.A. Study of three new halogenated oxoquinolinecarbohydrazide N-phosphonate derivatives as corrosion inhibitor for mild steel in acid environment. Surf. Interfaces 2020, 21, 100773. [Google Scholar] [CrossRef]
- Damej, M.; Kaya, S.; Ibrahimi, B.E.; Lee, H.-S.; Molhi, A.; Serdaroğlu, G.; Benmessaoud, M.; Ali, I.H.; Hajjaji, S.E.; Lgaz, H. The corrosion inhibition and adsorption behavior of mercaptobenzimidazole and bis-mercaptobenzimidazole on carbon steel in 1.0 M HCl: Experimental and computational insights. Surf. Interfaces 2021, 24, 101095. [Google Scholar] [CrossRef]
- Jodeh, S.; Larouj, M.; Lgaz, H.; Salghi, R.; Oudda, H.; Chetouani, A. Inhibitive Action of Sodium tetrafluoroborate on the Corrosion of Carbon Steel in Hydrochloric Acid Medieum. Moroc. J. Chem. 2016, 4, 425–436. [Google Scholar]
- Larouj, M.; Lgaz, H.; Salghi, R.; Jodeh, S.; Messali, M.; Zougagh, M.; Oudda, H.; Chetouani, A. Effect of chlorine group position on adsorption behavior and corrosion inhibition of Chlorobenzylideneamino-5-methyl-2,4-dihydro-1,2,4-triazole-3-thione Schiff bases: Experimental study. Moroc. J. Chem. 2016, 4, 567–583. [Google Scholar]
- Nanjan, M.J.; Mohammed, M.; Kumar, B.R.P.; Chandrasekar, M.J.N. Thiazolidinediones as antidiabetic agents: A critical review. Bioorganic Chem. 2018, 77, 548–567. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Thiazolidinediones: The forgotten diabetes medications. Curr. Diab. Rep. 2019, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, G.L.; Costa, S.N.; Freire, V.N.; Casciano, P.N.; Correia, A.N.; de Lima-Neto, P. Understanding the Corrosion Inhibition of Carbon Steel and Copper in Sulphuric Acid Medium by Amino Acids Using Electrochemical Techniques Allied to Molecular Modelling Methods. Corros. Sci. 2017, 115, 41–55. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, L.; Zhou, G. Inhibition of Copper Corrosion by Bis-(1-Benzotriazolymethylene)-(2,5-Thiadiazoly)-Disulfide in Chloride Media. Appl. Surf. Sci. 2004, 225, 287–293. [Google Scholar] [CrossRef]
- Yadav, M.; Behera, D.; Kumar, S.; Yadav, P. Experimental and Quantum Chemical Studies on Corrosion Inhibition Performance of Thiazolidinedione Derivatives for Mild Steel in Hydrochloric Acid Solution. Chem. Eng. Commun. 2015, 202, 303–315. [Google Scholar] [CrossRef]
- Lgaz, H.; Saha, S.K.; Lee, H.-S.; Kang, N.; Thari, F.Z.; Karrouchi, K.; Salghi, R.; Bougrin, K.; Ali, I.H. Corrosion Inhibition Properties of Thiazolidinedione Derivatives for Copper in 3.5 wt.% NaCl Medium. Metals 2021, 11, 1861. [Google Scholar] [CrossRef]
- Thari, F.Z.; Tachallait, H.; el Alaoui, N.-E.; Talha, A.; Arshad, S.; Álvarez, E.; Karrouchi, K.; Bougrin, K. Ultrasound-assisted one-pot green synthesis of new N- substituted-5-arylidene-thiazolidine-2,4-dione-isoxazoline derivatives using NaCl/Oxone/Na3PO4 in aqueous media. Ultrason. Sonochemistry 2020, 68, 105222. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, L.; Zhang, G.; Qiu, Y.; Guo, X. Benzotriazole as a Volatile Corrosion Inhibitor during the Early Stage of Copper Corrosion under Adsorbed Thin Electrolyte Layers. Corros. Sci. 2012, 65, 214–222. [Google Scholar] [CrossRef]
- Singh, P.; Ebenso, E.E.; Olasunkanmi, L.O.; Obot, I.B.; Quraishi, M. Electrochemical, theoretical, and surface morphological studies of corrosion inhibition effect of green naphthyridine derivatives on mild steel in hydrochloric acid. J. Phys. Chem. C. 2016, 120, 3408–3419. [Google Scholar] [CrossRef]
- Lutts, A.; Gielen, P. The precise determination of the lattice parameter of α-iron and some of its alloys. J. Appl. Crystallogr. 1971, 4, 242–250. [Google Scholar] [CrossRef]
- Guo, L.; Obot, I.B.; Zheng, X.; Shen, X.; Qiang, Y.; Kaya, S.; Kaya, C. Theoretical insight into an empirical rule about organic corrosion inhibitors containing nitrogen, oxygen, and sulfur atoms. Appl. Surf. Sci. 2017, 406, 301–306. [Google Scholar] [CrossRef]
- Guo, L.; Qi, C.; Zheng, X.; Zhang, R.; Shen, X.; Kaya, S. Toward understanding the adsorption mechanism of large size organic corrosion inhibitors on an Fe(110) surface using the DFTB method. RSC Adv. 2017, 7, 29042–29050. [Google Scholar] [CrossRef]
- Verma, C.; Olasunkanmi, L.O.; Ebenso, E.E.; Quraishi, M.A. Substituents effect on corrosion inhibition performance of organic compounds in aggressive ionic solutions: A review. J. Mol. Liq. 2018, 251, 100–118. [Google Scholar] [CrossRef]
- Khadom, A.A. Effect of temperature on corrosion inhibition of copper-nickel alloy by tetraethylenepentamine under flow conditions. J. Chil. Chem. Soc. 2014, 59, 2545–2549. [Google Scholar] [CrossRef]
- Desimone, M.; Gordillo, G.; Simison, S.N. The effect of temperature and concentration on the corrosion inhibition mechanism of an amphiphilic amido-amine in CO2 saturated solution. Corros. Sci. 2011, 53, 4033–4043. [Google Scholar] [CrossRef]
- Kairi, N.I.; Kassim, J. The effect of temperature on the corrosion inhibition of mild steel in 1 M HCl solution by Curcuma longa extract. Int. J. Electrochem. Sci. 2013, 8, 7138–7155. [Google Scholar]
- Elawady, Y.; Ahmed, A.I. Effect of Temperature & Inhibitors on the Corrosion of Aluminium in 2NHCI Solution: A Kinetic Study. Indian J. Chem. 1985, 24A, 601–602. [Google Scholar]
- Kubba, R.M.; Alag, A.S. Experimental and Theoretical Evaluation of new Quinazolinone Derivative as Organic Corrosion Inhibitor for Carbon Steel in 1M HCl Solution. Int. J. Sci. Res. 1832, 6, 1643. [Google Scholar]
- Lgaz, H.; Salghi, R.; Jodeh, S.; Hammouti, B. Effect of clozapine on inhibition of mild steel corrosion in 1.0 M HCl medium. J. Mol. Liq. 2017, 225, 271–280. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.-J.; Wang, Y.; Liu, Y.; Wu, Y.-C. Adsorption and corrosion inhibition properties of pyridine-2-aldehyde-2-quinolylhydrazone for Q235 steel in acid medium: Electrochemical, thermodynamic, and surface studies. Mater. Corros. 2018, 69, 1638–1648. [Google Scholar] [CrossRef]
- Chaouiki, A.; Lgaz, H.; Salghi, R.; Gaonkar, S.L.; Bhat, K.S.; Jodeh, S.; Toumiat, K.; Oudda, H. New Benzohydrazide Derivative as Corrosion Inhibitor for Carbon Steel in a 1.0 M HCl Solution: Electrochemical, DFT and Monte Carlo Simulation Studies. Port. Electrochimica Acta. 2019, 37, 147–165. [Google Scholar] [CrossRef]
- Selatnia, I.; Sid, A.; Benahmed, M.; Debbih, O.D.; Ozturk, T.; Gherraf, N. Synthesis and Characterization of a Bis-Pyrazoline Derivative as Corrosion Inhibitor for A283 Carbon Steel in 1M HCl: Electrochemical, Surface, DFT and MD Simulation Studies. Prot. Met. Phys. Chem. Surf. 2018, 54, 1182–1193. [Google Scholar] [CrossRef]
- Cao, Z.; Tang, Y.; Cang, H.; Xu, J.; Lu, G.; Jing, W. Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part II: Theoretical studies. Corros. Sci. 2014, 83, 292–298. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance spectroscopy and its use in analyzing the steady-state AC response of solid and liquid electrolytes. J. Electroanal. Chem. Interfacial Electrochem. 1987, 223, 25–50. [Google Scholar] [CrossRef]
- Avdeev, Y.G.; Kuznetsov, Y.I.; Buryak, A.K. Inhibition of steel corrosion by unsaturated aldehydes in solutions of mineral acids. Corros. Sci. 2013, 69, 50–60. [Google Scholar] [CrossRef]
- Ansari, K.R.; Quraishi, M.A.; Singh, A. Pyridine derivatives as corrosion inhibitors for N80 steel in 15% HCl: Electrochemical, surface and quantum chemical studies. Measurement 2015, 76, 136–147. [Google Scholar] [CrossRef]
- Chong, A.L.; Mardel, J.I.; MacFarlane, D.R.; Forsyth, M.; Somers, A.E. Synergistic corrosion inhibition of mild steel in aqueous chloride solutions by an imidazolinium carboxylate salt. ACS Sustain. Chem. Eng. 2016, 4, 1746–1755. [Google Scholar] [CrossRef]
- Abdallah, Z.A.; Ahmed, M.S.M.; Saleh, M. Organic synthesis and inhibition action of novel hydrazide derivative for mild steel corrosion in acid solutions. Mater. Chem. Phys. 2016, 174, 91–99. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, N.C.; Huang, X.D.; Shang, W.; Wu, L. Synergistic inhibition of carbon steel corrosion in 0.5 M HCl solution by indigo carmine and some cationic organic compounds: Experimental and theoretical studies. RSC Adv. 2016, 6, 22250–22268. [Google Scholar] [CrossRef]
- Gholami, M.; Danaee, I.; Maddahy, M.H.; RashvandAvei, M. Correlated ab initio and electroanalytical study on inhibition behavior of 2-mercaptobenzothiazole and its thiole–thione tautomerism effect for the corrosion of steel (API 5L X52) in sulphuric acid solution. Ind. Eng. Chem. Res. 2013, 52, 14875–14889. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochimica Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Aoun, S.B. On the corrosion inhibition of carbon steel in 1 M HCl with a pyridinium-ionic liquid: Chemical, thermodynamic, kinetic and electrochemical studies. RSC Adv. 2017, 7, 36688–36696. [Google Scholar] [CrossRef]
- Bayol, E.; Gürten, A.; Dursun, M.; Kayakirilmaz, K. Adsorption behavior and inhibition corrosion effect of sodium carboxymethyl cellulose on mild steel in acidic medium. Acta Phys. Chim. Sin. 2008, 24, 2236–2243. [Google Scholar] [CrossRef]
- Myung, N.V.; Park, D.-Y.; Yoo, B.-Y.; Sumodjo, P.T. Development of electroplated magnetic materials for MEMS. J. Magn. Magn. Mater. 2003, 265, 189–198. [Google Scholar] [CrossRef]
- Olasunkanmi, L.O.; Obot, I.B.; Ebenso, E.E. Adsorption and corrosion inhibition properties of N-{n-[1-R-5-(quinoxalin-6-yl)-4, 5-dihydropyrazol-3-yl] phenyl} methanesulfonamides on mild steel in 1 M HCl: Experimental and theoretical studies. RSC Adv. 2016, 6, 86782–86797. [Google Scholar] [CrossRef]
- Saha, S.K.; Dutta, A.; Ghosh, P.; Sukul, D.; Banerjee, P. Adsorption and corrosion inhibition effect of Schiff base molecules on the mild steel surface in 1 M HCl medium: A combined experimental and theoretical approach. Phys. Chem. Chem. Phys. 2015, 17, 5679–5690. [Google Scholar] [CrossRef]
- Fouda, A.; Diab, M.; Fathy, S. Role of Some Organic Compounds as Corrosion Inhibitors for 316L Stainless Steel in 1 M HCl. Int. J. Electrochem. Sci. 2017, 12, 347–362. [Google Scholar] [CrossRef]
- Chafiq, M.; Chaouiki, A.; Al-Hadeethi, M.R.; Salghi, R.; Ismat, H.A.; Shaaban, K.M.; Chung, I.-M. A joint experimental and theoretical investigation of the corrosion inhibition behavior and mechanism of hydrazone derivatives for mild steel in HCl solution. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125744. [Google Scholar] [CrossRef]
- Singh, P.; Srivastava, V.; Quraishi, M. Novel quinoline derivatives as green corrosion inhibitors for mild steel in acidic medium: Electrochemical, SEM, AFM, and XPS studies. J. Mol. Liq. 2016, 216, 164–173. [Google Scholar] [CrossRef]
- Kokalj, A. On the alleged importance of the molecular electron-donating ability and the HOMO–LUMO gap in corrosion inhibition studies. Corros. Sci. 2021, 180, 109016. [Google Scholar] [CrossRef]
- Kovačević, N.; Kokalj, A. Chemistry of the interaction between azole type corrosion inhibitor molecules and metal surfaces. Mater. Chem. Phys. 2012, 137, 331–339. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Haque, J.; Dohare, P.; Lgaz, H.; Salghi, R.; Quraishi, M.A. Effect of electron donating functional groups on corrosion inhibition of mild steel in hydrochloric acid: Experimental and quantum chemical study. J. Taiwan Inst. Chem. Eng. 2018, 82, 233–251. [Google Scholar] [CrossRef]
- Kumar, D.; Jain, N.; Jain, V.; Rai, B. Amino acids as copper corrosion inhibitors: A density functional theory approach. Appl. Surf. Sci. 2020, 514, 145905. [Google Scholar] [CrossRef]
- Kumar, D.; Jain, V.; Rai, B. Unravelling the mechanisms of corrosion inhibition of iron by henna extract: A density functional theory study. Corros. Sci. 2018, 142, 102–109. [Google Scholar] [CrossRef]
- Özcan, M.; Toffoli, D.; Üstünel, H.; Dehri, İ. Insights into surface–adsorbate interactions in corrosion inhibition processes at the molecular level. Corros. Sci. 2014, 80, 482–486. [Google Scholar] [CrossRef]
- Solmaz, R. Investigation of corrosion inhibition mechanism and stability of Vitamin B1 on mild steel in 0.5 M HCl solution. Corros. Sci. 2014, 81, 75–84. [Google Scholar] [CrossRef]
- Solmaz, R.; Şahin, E.A.; Döner, A.; Kardaş, G. The investigation of synergistic inhibition effect of rhodanine and iodide ion on the corrosion of copper in sulphuric acid solution. Corros. Sci. 2011, 53, 3231–3240. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, L.; She, Y. Insight on the corrosion inhibition performance of psidium guajava linn leaves extract. J. Mol. Liq. 2022, 346, 117858. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, A. Recent advances in metallic corrosion inhibition: A review. J. Mol. Liq. 2021, 322, 114862. [Google Scholar] [CrossRef]
- Shahmoradi, A.R.; Talebibahmanbigloo, N.; Nickhil, C.; Nisha, R.; Javidparvar, A.A.; Ghahremani, P.; Bahlakeh, G.; Ramezanzadeh, B. Molecular-MD/atomic-DFT theoretical and experimental studies on the quince seed extract corrosion inhibition performance on the acidic-solution attack of mild-steel. J. Mol. Liq. 2022, 346, 117921. [Google Scholar] [CrossRef]










| Medium | Concentration (mol/L) | Temperature (K) | Corrosion Rate (mg/cm2 × h) | Inhibition Efficiency (%) |
|---|---|---|---|---|
| Blank | 1.0 M HCl | 303 | 1.135 ± 0.0121 | - |
| 313 | 1.416 ± 0.0215 | - | ||
| 323 | 1.998 ± 0.0214 | - | ||
| 333 | 2.539 ± 0.0316 | - | ||
| MeOTZD | 5 × 10−3 | 303 | 0.0681 ± 0.0034 | 94 |
| 313 | 0.1274 ± 0.0071 | 91 | ||
| 323 | 0.2197 ± 0.0098 | 89 | ||
| 333 | 0.3808 ± 0.0029 | 85 | ||
| 1 × 10−3 | 303 | 0.1362 ± 0.0078 | 88 | |
| 313 | 0.2124 ± 0.0047 | 85 | ||
| 323 | 0.3396 ± 0.0098 | 83 | ||
| 333 | 0.4824 ± 0.0063 | 81 | ||
| 5 × 10−4 | 303 | 0.1816 ± 0.0027 | 84 | |
| 313 | 0.2549 ± 0.0067 | 81 | ||
| 323 | 0.3996 ± 0.0078 | 80 | ||
| 333 | 0.5586 ± 0.0088 | 78 | ||
| 1 × 10−4 | 303 | 0.227 ± 0.0026 | 80 | |
| 313 | 0.3115 ± 0.0043 | 78 | ||
| 323 | 0.4795 ± 0.0060 | 76 | ||
| 333 | 0.6855 ± 0.0079 | 73 |
| Medium | Concentration (mol/L) | Temperature (K) | Corrosion Rate (mg/cm2 × h) | Inhibition Efficiency (%) |
|---|---|---|---|---|
| Blank | 1.0 | 303 | 1.135 ± 0.0121 | - |
| 313 | 1.416 ± 0.0215 | - | ||
| 323 | 1.998 ± 0.0214 | - | ||
| 333 | 2.539 ± 0.0316 | - | ||
| MeTZD | 5 × 10−3 | 303 | 0.187 ± 0.0034 | 83 |
| 313 | 0.297 ± 0.0032 | 79 | ||
| 323 | 0.539 ± 0.0067 | 73 | ||
| 333 | 0.863 ± 0.0089 | 66 | ||
| 1 × 10−3 | 303 | 0.238 ± 0.0054 | 79 | |
| 313 | 0.368 ± 0.0035 | 74 | ||
| 323 | 0.619 ± 0.0078 | 69 | ||
| 333 | 0.990 ± 0.0084 | 61 | ||
| 5 × 10−4 | 303 | 0.295 ± 0.0098 | 74 | |
| 313 | 0.439 ± 0.0045 | 69 | ||
| 323 | 0.719 ± 0.0067 | 64 | ||
| 333 | 1.117 ± 0.0089 | 56 | ||
| 1 × 10−4 | 303 | 0.355 ± 0.0043 | 69 | |
| 313 | 0.467 ± 0.0065 | 65 | ||
| 323 | 0.794 ± 0.0078 | 60 | ||
| 333 | 1.193 ± 0.0085 | 53 |
| Inhibitor | Concentration (mol/L) | −Ecorr (mV vs. SCE) | −βc (mV dec−1) | icorr (μAcm−2) | (%) |
|---|---|---|---|---|---|
| Blank | 1.0 | 496 ± 0.4 | 150 ± 3.5 | 599 ± 2.4 | - |
| MeOTZD | 5 × 10−3 | 480 ± 0.6 | 203 ± 0.6 | 29.9 ± 0.8 | 95 |
| 1 × 10−3 | 490 ± 0.5 | 190 ± 0.4 | 59.9 ± 0.8 | 90 | |
| 5 × 10−4 | 496 ± 0.7 | 183 ± 0.6 | 83.8 ± 0.5 | 86 | |
| 1 × 10−4 | 501 ± 0.9 | 160 ± 1.2 | 113.8 ± 0.9 | 81 | |
| MeTZD | 5 × 10−3 | 502 ± 0.8 | 181 ± 1.1 | 77.8 ± 1.3 | 87 |
| 1 × 10−3 | 508 ± 1.4 | 170 ± 0.9 | 101.8 ± 0.2 | 83 | |
| 5 × 10−4 | 480 ± 1.3 | 159 ± 0.6 | 131.8 ± 1.1 | 78 | |
| 1 × 10−4 | 496 ± 0.5 | 150 ± 0.7 | 155.7 ± 0.6 | 74 |
| Inhibitor | Concentration (mol/L) | (%) | ||||
|---|---|---|---|---|---|---|
| Blank | 1.0 | 25.03 ± 1.3 | 0.90123 ± 0.008 | 1.772 ± 0.0018 | 97 | |
| MeOTZD | 654.7 ± 1.9 | 0.78276 ± 0.009 | 0.220 ± 0.0043 | 6 | 96 | |
| 275.6 ± 1.6 | 0.78004 ± 0.004 | 0.450 ± 0.0066 | 13 | 91 | ||
| 180.8 ± 1.5 | 0.82262 ± 0.008 | 0.570 ± 0.0023 | 21 | 86 | ||
| 139.7 ± 1.3 | 0.84478 ± 0.002 | 0.600 ± 0.0067 | 24 | 82 | ||
| MeTZD | 217.1 ± 1.7 | 0.72809 ± 0.001 | 0.450 ± 0.0089 | 8 | 88 | |
| 150.1 ± 0.8 | 0.78788 ± 0.006 | 0.550 ± 0.0054 | 15 | 83 | ||
| 120.2 ± 0.5 | 0.76825 ± 0.005 | 0.810 ± 0.0036 | 20 | 79 | ||
| 98.22 ± 1.4 | 0.79927 ± 0.009 | 0.910 ± 0.0078 | 27 | 74 |
| System | Linear Polarization Data | ||
|---|---|---|---|
| Concentration (mol/L) | Rp (Ω cm2) | ηLPR (%) | |
| HCl | 1.0 | 28 ± 0.9 | - |
| MeOTZD | 5 × 10−3 1 × 10−3 5 × 10−4 1 × 10−4 | 731.0 ± 0.5 308.0 ± 0.8 202.1 ± 0.3 156.2 ± 1.6 | 96 91 86 82 |
| MeTZD | 5 × 10−3 1 × 10−3 5 × 10−4 1 × 10−4 | 242.8 ± 0.9 167.8 ± 1.1 134.4 ± 0.6 109.8 ± 1.4 | 88 83 79 75 |
| Inhibitor | Temperature (K) | Kads (L/mol) | R2 | (KJ/mol) | (KJ/mol−1) | (J mol−1 K−1) |
|---|---|---|---|---|---|---|
| MeTZD | 303 | 17,317 | 0.999 | −34.70 | - | - |
| MeOTZD | 303 | 34,450 | 0.999 | −36.44 | −69.56 | 99 |
| 313 | 17,335 | 0.999 | −37.00 | |||
| 323 | 33,673 | 0.999 | −37.58 | |||
| 333 | 36,948 | 0.999 | −40.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaouiki, A.; Chafiq, M.; Ko, Y.G.; Al-Moubaraki, A.H.; Thari, F.Z.; Salghi, R.; Karrouchi, K.; Bougrin, K.; Ali, I.H.; Lgaz, H. Adsorption Mechanism of Eco-Friendly Corrosion Inhibitors for Exceptional Corrosion Protection of Carbon Steel: Electrochemical and First-Principles DFT Evaluations. Metals 2022, 12, 1598. https://doi.org/10.3390/met12101598
Chaouiki A, Chafiq M, Ko YG, Al-Moubaraki AH, Thari FZ, Salghi R, Karrouchi K, Bougrin K, Ali IH, Lgaz H. Adsorption Mechanism of Eco-Friendly Corrosion Inhibitors for Exceptional Corrosion Protection of Carbon Steel: Electrochemical and First-Principles DFT Evaluations. Metals. 2022; 12(10):1598. https://doi.org/10.3390/met12101598
Chicago/Turabian StyleChaouiki, Abdelkarim, Maryam Chafiq, Young Gun Ko, Aisha H. Al-Moubaraki, Fatima Zahra Thari, Rachid Salghi, Khalid Karrouchi, Khalid Bougrin, Ismat H. Ali, and Hassane Lgaz. 2022. "Adsorption Mechanism of Eco-Friendly Corrosion Inhibitors for Exceptional Corrosion Protection of Carbon Steel: Electrochemical and First-Principles DFT Evaluations" Metals 12, no. 10: 1598. https://doi.org/10.3390/met12101598