The Role of Graphitic Carbon Nitride in the Formulation of Copper-Free Friction Composites Designed for Automotive Brake Pads
Abstract
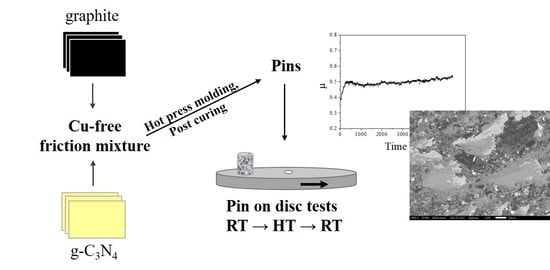
:1. Introduction
2. Materials and Methods
2.1. Master Batch and Graphite
2.2. Graphitic Carbon Nitride
2.3. Modification of the Master Batch with gCN and G
- 9 wt.% of G: formulation labelled as M0_G;
- 9 wt.% of gCN: formulation labelled as M0_CN.
2.4. Characterisation of the Thermal Stability of gCN, G, and the Prepared Friction Composites
2.5. Pin-On-Disc Tests
3. Results and Discussion
3.1. Characterisation of gCN
3.2. Thermal Stability of the Friction Composites
3.3. Friction and Wear Behaviour at RT
3.4. Friction and Wear Behaviour of the Samples at HT and Recovery of Their Friction-Wear Performance
3.5. Analysis of Worn Surfaces
3.6. Discussion on the Role of Studied Lubricants on Friction-Wear Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cox, R.L. Engineered Tribological Composites: The Art of Friction Material Development; SAE International: Warrendale, PA, USA, 2011; p. 524. [Google Scholar]
- Xiao, Y.; Yao, P.; Zhou, H.; Zhang, Z.; Gong, T.; Zhao, L.; Deng, M. Investigation on Speed-Load Sensitivity to Tribological Properties of Copper Metal Matrix Composites for Braking Application. Metals 2020, 10, 889. [Google Scholar] [CrossRef]
- Lyu, Y.; Leonardi, M.; Mancini, A.; Wahlström, J.; Olofsson, U. Tribology and Airborne Particle Emission of Laser-Cladded Fe-Based Coatings versus Non-Asbestos Organic and Low-Metallic Brake Materials. Metals 2021, 11, 1703. [Google Scholar] [CrossRef]
- Seo, H.; Park, J.; Kim, Y.C.; Lee, J.J.; Jang, H. Effect of disc materials on brake emission during moderate-temperature braking. Tribol. Int. 2021, 163, 107185. [Google Scholar] [CrossRef]
- Matějka, V.; Metinöz, I.; Wahlström, J.; Alemani, M.; Perricone, G. On the running-in of brake pads and discs for dyno bench tests. Tribol. Int. 2017, 115, 424–431. [Google Scholar] [CrossRef]
- Chan, D.; Stachowiak, G.W. Review of automotive brake friction materials. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2004, 218, 953–966. [Google Scholar] [CrossRef]
- Fan, Y.; Matějka, V.; Kratošová, G.; Lu, Y. Role of Al2O3 in Semi-Metallic Friction Materials and its Effects on Friction and Wear Performance. Tribol. Trans. 2008, 51, 771–778. [Google Scholar] [CrossRef]
- Matejka, V.; Lu, Y.F.; Jiao, L.; Huang, L.; Martynkova, G.S.; Tomasek, V. Effects of silicon carbide particle sizes on friction-wear properties of friction composites designed for car brake lining applications. Tribol. Int. 2010, 43, 144–151. [Google Scholar] [CrossRef]
- Ma, Y.; Martynková, G.S.; Valášková, M.; Matějka, V.; Lu, Y. Effects of ZrSiO4 in non-metallic brake friction materials on friction performance. Tribol. Int. 2008, 41, 166–174. [Google Scholar] [CrossRef]
- Park, J.; Song, W.; Gweon, J.; Seo, H.; Lee, J.J.; Jang, H. Size effect of zircon particles in brake pads on the composition and size distribution of emitted particulate matter. Tribol. Int. 2021, 160, 106995. [Google Scholar] [CrossRef]
- Aranganathan, N.; Bijwe, J. Special grade of graphite in NAO friction materials for possible replacement of copper. Wear 2015, 330, 515–523. [Google Scholar] [CrossRef]
- Matějka, V.; Lu, Y.; Matějková, P.; Smetana, B.; Kukutschová, J.; Vaculík, M.; Tomášek, V.; Zlá, S.; Fan, Y. Possible stibnite transformation at the friction surface of the semi-metallic friction composites designed for car brake linings. Appl. Surf. Sci. 2011, 258, 1862–1868. [Google Scholar] [CrossRef] [Green Version]
- Österle, W.; Dmitriev, A.I. The Role of Solid Lubricants for Brake Friction Materials. Lubricants 2016, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.-Y.; Cheng, H.-Z.; Lee, K.-J.; Wang, C.-F.; Liu, Y.-C.; Wang, Y.-W. Effect of Carbonaceous Components on Tribological Properties of Copper-Free NAO Friction Material. Materials 2020, 13, 1163. [Google Scholar] [CrossRef] [Green Version]
- Matějka, V.; Fu, Z.; Kukutschová, J.; Qi, S.; Jiang, S.; Zhang, X.; Yun, R.; Vaculík, M.; Heliová, M.; Lu, Y. Jute fibers and powderized hazelnut shells as natural fillers in non-asbestos organic non-metallic friction composites. Mater. Des. 2013, 51, 847–853. [Google Scholar] [CrossRef]
- Baklouti, M.; Cristol, A.L.; Desplanques, Y.; Elleuch, R. Impact of the glass fibers addition on tribological behavior and braking performances of organic matrix composites for brake lining. Wear 2015, 330, 507–514. [Google Scholar] [CrossRef]
- Matějka, V.; Perricone, G.; Vlček, J.; Olofsson, U.; Wahlström, J. Airborne Wear Particle Emissions Produced during the Dyno Bench Tests with a Slag Containing Semi-Metallic Brake Pads. Atmosphere 2020, 11, 1220. [Google Scholar] [CrossRef]
- Handa, Y.; Kato, T. Effects of Cu Powder, BaSO4 and Cashew Dust on the Wear and Friction Characteristics of Automotive Brake Pads. Tribol. Trans. 1996, 39, 346–353. [Google Scholar] [CrossRef]
- Österle, W.; Prietzel, C.; Kloß, H.; Dmitriev, A.I. On the role of copper in brake friction materials. Tribol. Int. 2010, 43, 2317–2326. [Google Scholar] [CrossRef]
- Hong, U.S.; Jung, S.L.; Cho, K.H.; Cho, M.H.; Kim, S.J.; Jang, H. Wear mechanism of multiphase friction materials with different phenolic resin matrices. Wear 2009, 266, 739–744. [Google Scholar] [CrossRef]
- Jara, D.C.; Jang, H. Synergistic effects of the ingredients of brake friction materials on friction and wear: A case study on phenolic resin and potassium titanate. Wear 2019, 430, 222–232. [Google Scholar] [CrossRef]
- Abutu, J.; Lawal, S.A.; Ndaliman, M.B.; Lafia-Araga, R.A.; Adedipe, O.; Choudhury, I.A. Effects of process parameters on the properties of brake pad developed from seashell as reinforcement material using grey relational analysis. Eng. Sci. Technol. Int. J. 2018, 21, 787–797. [Google Scholar] [CrossRef]
- Cho, M.H.; Kim, S.J.; Kim, D.; Jang, H. Effects of ingredients on tribological characteristics of a brake lining: An experimental case study. Wear 2005, 258, 1682–1687. [Google Scholar] [CrossRef]
- Surya Rajan, B.; Sai Balaji, M.A.; Mohamed Aslam Noorani, A.B.M.A. Tribological performance of graphene/graphite filled phenolic composites-A comparative study. Compos. Commun. 2019, 15, 34–39. [Google Scholar]
- Bijwe, J.; Aranganathan, N.; Sharma, S.; Dureja, N.; Kumar, R. Nano-abrasives in friction materials-influence on tribological properties. Wear 2012, 296, 693–701. [Google Scholar] [CrossRef]
- Mahale, V.; Bijwe, J.; Sinha, S. Influence of nano-potassium titanate particles on the performance of NAO brake-pads. Wear 2017, 376, 727–737. [Google Scholar] [CrossRef]
- Antonyraj, I.J.; Singaravelu, D.L. Tribological characterization of various solid lubricants based copper-free brake friction materials–A comprehensive study. Mater. Today Proc. 2020, 27, 2650–2656. [Google Scholar] [CrossRef]
- Kalel, N.; Bhatt, B.; Darpe, A.; Bijwe, J. Copper-free brake-pads: A break-through by selection of the right kind of stainless steel particles. Wear 2021, 464, 203537. [Google Scholar] [CrossRef]
- Aranganathan, N.; Bijwe, J. Development of copper-free eco-friendly brake-friction material using novel ingredients. Wear 2016, 352, 79–91. [Google Scholar] [CrossRef]
- Bhatt, B.; Kalel, N.; Darpe, A.; Bijwe, J. Role of Promaxon-D in Controlling Tribological Performance of Cu-Free Brake Pads. Metals 2021, 11, 441. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Yin, L.; Cheng, R.; Song, Q.; Yang, J.; Kong, X.; Huang, J.; Lin, Y.; Ouyang, H. Construction of nanoflower SnS2 anchored on g-C3N4 nanosheets composite as highly efficient anode for lithium ion batteries. Electrochim. Acta 2019, 293, 408–418. [Google Scholar] [CrossRef]
- Das, D.; Shinde, S.; Nanda, K. Temperature-dependent photoluminescence of g-C3N4: Implication for temperature sensing. ACS Appl. Mater. Interfaces 2016, 8, 2181–2186. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Tsumura, T.; Kinumoto, T.; Toyoda, M. Graphitic carbon nitrides (g-C3N4) with comparative discussion to carbon materials. Carbon 2019, 141, 580–607. [Google Scholar] [CrossRef]
- Reddy, K.R.; Reddy, C.H.V.; Nadagouda, M.N.; Shetti, N.P.; Jaesool, S.; Aminabhavi, T.M. Polymeric graphitic carbon nitride (g-C3N4)-based semiconducting nanostructured materials: Synthesis methods, properties and photocatalytic applications. J. Environ. Manag. 2019, 238, 25–40. [Google Scholar] [CrossRef]
- Praus, P.; Svoboda, L.; Ritz, M.; Troppová, I.; Šihor, M.; Kočí, K. Graphitic carbon nitride: Synthesis, characterization and photocatalytic decomposition of nitrous oxide. Mater. Chem. Phys. 2017, 193, 438–446. [Google Scholar] [CrossRef]
- Zhu, L.; You, L.; Shi, Z.; Song, H.; Li, S. An investigation on the graphitic carbon nitride reinforced polyimide composite and evaluation of its tribological properties. J. Appl. Polym. Sci. 2017, 134, 45403. [Google Scholar] [CrossRef]
- Duan, C.; Yuan, D.; Yang, Z.; Li, S.; Tao, L.; Wang, Q.; Wang, T. High wear-resistant performance of thermosetting polyimide reinforced by graphitic carbon nitride (g-C3N4) under high temperature. Compos. Part A 2018, 113, 200–208. [Google Scholar] [CrossRef]
- Zhang, L.; Li, G.; Guo, Y.; Qi, H.; Che, Q.; Zhang, G. PEEK reinforced with low-loading 2D graphitic carbon nitride nanosheets: High wear resistance under harsh lubrication conditions. Compos. Part A 2018, 109, 507–516. [Google Scholar] [CrossRef]
- Leonardi, M.; Alemani, M.; Straffelini, G.; Gialanella, S. A pin-on-disc study on the dry sliding behavior of a Cu-free friction material containing different types of natural graphite. Wear 2020, 442, 203157. [Google Scholar] [CrossRef]
- Praus, P.; Smykalova, A.; Fonioka, K.; Matejka, V.; Kormunda, M.; Smetana, B.; Cvejn, D. The presence and effect of oxygen in graphitic carbon nitride synthetized in air and nitrogen atmosphere. Appl. Surf. Sci. 2020, 529, 10. [Google Scholar] [CrossRef]
- Nasir, M.S.; Yang, G.; Ayub, I.; Wang, S.; Wang, L.; Wang, X.; Yan, W.; Peng, S.; Ramakarishna, S. Recent development in graphitic carbon nitride based photocatalysis for hydrogen generation. Appl. Catal. B 2019, 257, 117855. [Google Scholar] [CrossRef]
- Pan, H.; Zhang, H.; Liu, H.; Chen, L. Interstitial boron doping effects on the electronic and magnetic properties of graphitic carbon nitride materials. Solid State Commun. 2015, 203, 35–40. [Google Scholar] [CrossRef]
- Han, Q.; Hu, C.; Zhao, F.; Zhang, Z.; Chen, N.; Qu, L. One-step preparation of iodine-doped graphitic carbon nitride nanosheets as efficient photocatalysts for visible light water splitting. J. Mater. Chem. A 2015, 3, 4612–4619. [Google Scholar] [CrossRef]
- Dong, G.; Ai, Z.; Zhang, L. Efficient anoxic pollutant removal with oxygen functionalized graphitic carbon nitride under visible light. RSC Adv. 2014, 4, 5553–5560. [Google Scholar] [CrossRef]
- Leonardi, M.; Menapace, C.; Matějka, V.; Gialanella, S.; Straffelini, G. Pin-on-disc investigation on copper-free friction materials dry sliding against cast iron. Tribol. Int. 2018, 119, 73–81. [Google Scholar] [CrossRef]
- Verma, P.C.; Ciudin, R.; Bonfanti, A.; Aswath, P.; Straffelini, G.; Gialanella, S. Role of the friction layer in the high-temperature pin-on-disc study of a brake material. Wear 2016, 346, 56–65. [Google Scholar] [CrossRef]
- Menapace, C.; Leonardi, M.; Secchi, M.; Bonfanti, A.; Gialanella, S.; Straffelini, G. Thermal behavior of a phenolic resin for brake pad manufacturing. J. Therm. Anal. Calorim. 2019, 137, 759–766. [Google Scholar] [CrossRef]
- Matějka, V.; Lu, Y.; Fan, Y.; Kratošová, G.; Lešková, J. Effects of silicon carbide in semi-metallic brake materials on friction performance and friction layer formation. Wear 2008, 265, 1121–1128. [Google Scholar] [CrossRef]
- Lyu, Y.; Leonardi, M.; Wahlström, J.; Gialanella, S.; Olofsson, U. Friction, wear and airborne particle emission from Cu-free brake materials. Tribol. Int. 2020, 141, 105959. [Google Scholar] [CrossRef]
- Kumar, M.; Bijwe, J. Non-asbestos organic (NAO) friction composites: Role of copper; its shape and amount. Wear 2011, 270, 269–280. [Google Scholar] [CrossRef]
- Menapace, C.; Leonardi, M.; Matějka, V.; Gialanella, S.; Straffelini, G. Dry sliding behavior and friction layer formation in copper-free barite containing friction materials. Wear 2018, 398, 191–200. [Google Scholar] [CrossRef]









| Constituents of M0 | Main Role | Content (wt.%) |
|---|---|---|
| Steel | Reinforcing fibres | 30 |
| Aluminium oxide, silicon carbide, and magnesium oxide | Abrasives | 25 |
| Tin sulphide, sphalerite, and zinc oxide | Lubricants | 13 |
| Vermiculite and others | Fillers | 24 |
| Phenolic resin | Binder | 8 |
| Fraction content | 0.4% | 23% | 37% | 72% | 91% |
| Fraction size | >800 µm | >600 µm | >550 µm | >250 µm | >150 µm |
| Elements | C | N | Al | O |
| Content (wt.%) | 44.9 | 41.1 | 12.1 | 1.9 |
| Sample | µ (-) | Ka (×10−14 m2/N) | ||||
|---|---|---|---|---|---|---|
| RT | HT | H_RT | RT | HT | H_RT | |
| M0 | 0.50 ± 0.04 | 0.38 ± 0.01 | 0.43 ± 0.06 | 6.56 ± 1.22 | 10.44 ± 0.93 | 15.65 ± 2.19 |
| M0_G | 0.46 ± 0.01 | 0.37 ± 0.01 | 0.39 ± 0.02 | 3.85 ± 0.37 | 8.02 ± 0.52 | 4.44 ± 1.58 |
| M0_CN | 0.52 ± 0.01 | 0.37 ± 0.04 | 0.49 ± 0.04 | 6.57 ± 0.08 | 11.10 ± 0.21 | 12.20 ± 0.42 |
| Element (wt.%) | M0 | M0_G | M0_CN |
|---|---|---|---|
| Fe | 60.19 | 60.0 | 54.76 |
| C | 6.72 | 8.91 | 9.73 |
| Zn | 4.41 | 3.59 | 4.94 |
| Al | 2.12 | 2.21 | 2.23 |
| Mg | 2.12 | 2.27 | 2.26 |
| Sn | 2.06 | 2.54 | 2.98 |
| S | 1.84 | 2.38 | 2.70 |
| Si | 1.23 | 1.36 | 1.02 |
| N | - | - | 1.18 |
| Cr | 0.96 | 0.98 | 0.88 |
| Ca | 0.71 | 0.81 | 0.92 |
| O | 17.67 | 14.93 | 16.42 |
| Content Wt.% | Point | |
|---|---|---|
| 01 | 02 | |
| Si | 57.61 | - |
| C | 28.46 | 5.64 |
| Fe | 12.07 | 93.59 |
| O | 1.86 | - |
| N | - | 0.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matějka, V.; Leonardi, M.; Praus, P.; Straffelini, G.; Gialanella, S. The Role of Graphitic Carbon Nitride in the Formulation of Copper-Free Friction Composites Designed for Automotive Brake Pads. Metals 2022, 12, 123. https://doi.org/10.3390/met12010123
Matějka V, Leonardi M, Praus P, Straffelini G, Gialanella S. The Role of Graphitic Carbon Nitride in the Formulation of Copper-Free Friction Composites Designed for Automotive Brake Pads. Metals. 2022; 12(1):123. https://doi.org/10.3390/met12010123
Chicago/Turabian StyleMatějka, Vlastimil, Mara Leonardi, Petr Praus, Giovanni Straffelini, and Stefano Gialanella. 2022. "The Role of Graphitic Carbon Nitride in the Formulation of Copper-Free Friction Composites Designed for Automotive Brake Pads" Metals 12, no. 1: 123. https://doi.org/10.3390/met12010123