Comparative Study for Selective Lithium Recovery via Chemical Transformations during Incineration and Dynamic Pyrolysis of EV Li-Ion Batteries
Abstract
:1. Introduction
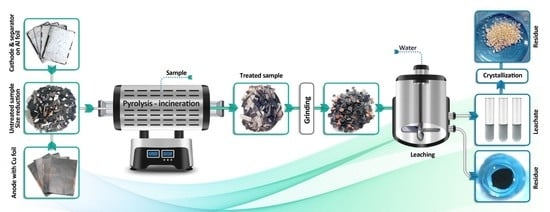
2. Materials and Methods
2.1. Thermal Treatment
2.2. Chemicals Used
2.3. Analytical Techniques
2.4. Leaching Experiment and Analytical Procedure
2.5. Evaporative Crystallization
3. Results
3.1. Elemental Composition of Solid Samples
3.2. The Effect of Thermal Treatment on Li Leaching Efficiency
3.3. The Effect of L/S Ratio on the Leaching Efficiency of Li
3.4. The Effect of Leaching Temperature on the Leaching Efficiency of Li
3.5. Effect of Addition of Excess Carbon
3.6. Leaching of Al
3.7. Effect of Thermal Treatment Temperature on the Leaching Efficiency of Al
3.8. Effect of L/S Ratio on the Leaching Efficiency of Al
3.9. Effect of Leaching Temperature on the Leaching Efficiency of Al
3.10. Effect of Addition of Excess Carbon
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drabik, E.; Rizos, V. Prospects for electric vehicle batteries in a circular economy. CEPS Res. Rep. 2018, 20. [Google Scholar]
- Li, J.; Wang, G.; Xu, Z. Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries. J. Hazard. Mater. 2016, 302, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Söderman, M.L.; Kushnir, D.; Sandén, B. Will metal scarcity limit the use of electric vehicles? Syst. Perspect. Electromobility 2013, 76–89. [Google Scholar]
- Peng, C.; Liu, W.; Wang, Z.; Wilson, B.P.; Lundström, M. Selective extraction of lithium (Li) and preparation of battery grade lithium carbonate (Li2CO3) from spent Li-ion batteries in nitrate system. J. Power Sources 2019, 415, 179–188. [Google Scholar] [CrossRef]
- Greim, P.; Solomon, A.A.; Breyer, C. Assessment of lithium criticality in the global energy transition and addressing policy gaps in transportation. Nat. Commun. 2020, 11, 4570. [Google Scholar] [CrossRef] [PubMed]
- Agusdinata, D.B.; Liu, W.; Eakin, H.; Romero, H. Socio-environmental impacts of lithium mineral extraction: Towards a research agenda. Environ. Res. Lett. 2018, 13, 123001. [Google Scholar] [CrossRef] [Green Version]
- Wanger, T.C. The Lithium future—Resources, recycling, and the environment. Conserv. Lett. 2011, 4, 202–206. [Google Scholar] [CrossRef]
- European Commission. Study on the EU’s List of Critical Raw Materials—Final Report (2020); European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Graedel, T.E.; Allwood, J.; Birat, J.P.; Reck, B.K.; Sibley, S.F.; Sonnemann, G.; Buchert, M.; Hageluken, C. Recycling Rates of Metals—A Status Report. A Report of the Working Group on the Global Metal Flow to the International Resource Panel; UNEP: Nairobi, Kenya, 2011. [Google Scholar]
- Zhao, Y.; Liu, B.; Zhang, L.; Guo, S. Microwave-absorbing properties of cathode material during reduction roasting for spent lithium-ion battery recycling. J. Hazard. Mater. 2020, 384, 121487. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Li, J.; Xu, Z. Novel approach for in situ recovery of lithium carbonate from spent lithium ion batteries using vacuum metallurgy. Environ. Sci. Technol. 2017, 51, 11960–11966. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, G. Effects of Pyrolysis and Incineration on the Chemical Composition of Li-Ion Batteries and Analysis of the by-Products. Ph.D. Thesis, Chalmers University of Technology, Goteborg, Sweden, 2019. [Google Scholar]
- Ekberg, C.; Petranikova, M. Lithium Batteries Recycling. In Lithium Process Chemistry; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 233–267. ISBN 978-0-12-801417-2. [Google Scholar]
- Kuzuhara, S.; Ota, M.; Tsugita, F.; Kasuya, R. Recovering lithium from the cathode active material in lithium-ion batteries via thermal decomposition. Metals 2020, 10, 433. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Xiao, L.; Tang, Y.; Chen, Y.; Ye, L.; Zhu, Y. Study on the reduction roasting of spent LiNixCoyMnzO2 lithium-ion battery cathode materials. J. Therm. Anal. Calorim. 2019, 136, 1323–1332. [Google Scholar] [CrossRef]
- Jandova, J.; Dvorak, P.; Kondas, J.; Havlak, L. Recovery of lithium from waste materials. Ceram. Silikáty 2012, 56, 50–54. [Google Scholar]
- Paulino, J.F.; Busnardo, N.G.; Afonso, J.C. Recovery of valuable elements from spent Li-batteries. J. Hazard. Mater. 2008, 150, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Lide, D.R. CRC Handbook of Chemistry and Physics; Internet Version 2005; CRC Press: Boca Raton, FL, USA, 2005; Available online: http://www.hbcpnetbase.com (accessed on 12 December 2020).
- Xue, S.; Zhang, L.; Han, Z.; Huang, X. Reaction mechanism between oxide film on surface of Al-Li alloy and CsF-AlF3 flux. Trans. Nonferrrous Met. Soc. China 2008, 18, 121–125. [Google Scholar] [CrossRef]
- Schwich, L.; Schubert, T.; Friedrich, B. Early-Stage Recovery of Lithium from Tailored Thermal Conditioned Black Mass Part I: Mobilizing Lithium via Supercritical CO2-Carbonation. Metals 2021, 11, 177. [Google Scholar] [CrossRef]
- Zheng, J.M.; Zhang, Z.R.; Xu, X.B.; Dong, Z.X.; Zhu, Z.; Yang, Y. The effect of AlF3 coating on the performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 positive electrode material for lithium-ion battery. J. Electrochem. Soc. 2008, 155, A775–A782. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. Functional behavior of AlF3 coatings for high-performance cathode materials for lithium-ion batteries. AIMS Mater. Sci. 2019, 6, 406–440. [Google Scholar] [CrossRef]
- Vargel, C. Inorganic Acids. In Corrosion of Aluminium; Elsevier: Amsterdam, The Netherlands, 2004; pp. 397–414. ISBN 9780080444956. [Google Scholar] [CrossRef]
- Cano, A.M.; Marquardt, A.E.; DuMont, J.M.; George, S.M. Effect of HF pressure on Thermal Al2O3 atomic layer etch rates and Al2O3 fluorination. J. Phys. Chem. 2019, 123, 10346–10355. [Google Scholar] [CrossRef]









| Treatment | Temperature (°C) | Time (min) | Li (wt.%) | Al (wt.%) |
|---|---|---|---|---|
| Untreated | 2.2 ± 0.2 | 6.2±0.3 | ||
| Incineration | 400 | 30 | 2.6 ± 0.2 | 5.9 ± 1.0 |
| 60 | 2.7 ± 0.1 | 6.0 ± 0.7 | ||
| 90 | 3.3 ± 0.1 | 9.8 ± 0.2 | ||
| 500 | 30 | 3.2 ± 0.2 | 11.6 ± 0.3 | |
| 60 | 3.1 ± 0.1 | 11.4 ± 0.5 | ||
| 90 | 2.4 ± 0.2 | 9.2 ± 0.9 | ||
| 600 | 30 | 2.0 ± 0.0 | 8.2 ± 0.3 | |
| 60 | 2.8 ± 0.2 | 10.0 ± 0.2 | ||
| 90 | 2.6 ± 0.1 | 10.3 ± 1.1 | ||
| 700 | 30 | 3.3 ± 0.0 | 10.1 ± 0.9 | |
| 60 | 3.7 ± 0.0 | 8.9 ± 0.3 | ||
| 90 | 3.6 ± 0.1 | 9.8 ± 0.3 | ||
| Pyrolysis | 400 | 30 | 2.6 ± 0.0 | 8.5 ± 0.5 |
| 60 | 2.3 ± 0.1 | 9.2 ± 0.9 | ||
| 90 | 2.6 ± 0.1 | 9.0 ± 1.1 | ||
| 500 | 30 | 3.0 ± 0.0 | 8.6 ± 0.3 | |
| 60 | 2.8 ± 1.1 | 7.8 ± 0.7 | ||
| 90 | 2.7 ± 0.0 | 9.3 ± 0.9 | ||
| 600 | 30 | 2.8 ± 0.0 | 8.6 ± 0.6 | |
| 60 | 2.9 ± 0.2 | 8.7 ± 1.1 | ||
| 90 | 2.6 ± 0.0 | 10.3 ± 0.8 | ||
| 700 | 30 | 2.9 ± 0.0 | 10.3 ± 0.9 | |
| 60 | 3.0 ± 0.1 | 9.1 ± 0.4 | ||
| 90 | 2.5 ± 0.1 | 10.2 ± 0.7 | ||
| Pyrolysis with additional carbon | Untreated | 3.4 ± 0.7 | 8.9 ± 0.5 | |
| 400 | 30 | 3.1 ± 0.1 | 5.9 ± 0.8 | |
| 500 | 30 | 3.2 ± 0.0 | 6.0 ± 0.0 | |
| 600 | 30 | 3.0 ± 0.4 | 6.4 ± 0.6 | |
| 700 | 30 | 3.1 ± 0.2 | 8.6 ± 1.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balachandran, S.; Forsberg, K.; Lemaître, T.; Vieceli, N.; Lombardo, G.; Petranikova, M. Comparative Study for Selective Lithium Recovery via Chemical Transformations during Incineration and Dynamic Pyrolysis of EV Li-Ion Batteries. Metals 2021, 11, 1240. https://doi.org/10.3390/met11081240
Balachandran S, Forsberg K, Lemaître T, Vieceli N, Lombardo G, Petranikova M. Comparative Study for Selective Lithium Recovery via Chemical Transformations during Incineration and Dynamic Pyrolysis of EV Li-Ion Batteries. Metals. 2021; 11(8):1240. https://doi.org/10.3390/met11081240
Chicago/Turabian StyleBalachandran, Srija, Kerstin Forsberg, Tom Lemaître, Nathália Vieceli, Gabriele Lombardo, and Martina Petranikova. 2021. "Comparative Study for Selective Lithium Recovery via Chemical Transformations during Incineration and Dynamic Pyrolysis of EV Li-Ion Batteries" Metals 11, no. 8: 1240. https://doi.org/10.3390/met11081240