Microstructure and Hardness Evolution of Al8Zn7Ni3Mg Alloy after Casting at very Different Cooling Rates
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Computational Section
3.2. Solidification Path and Structure Analysis
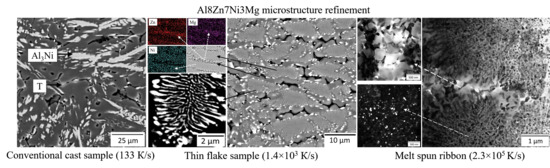
3.3. Characterization of the Quasi-Eutectic Structure
3.4. Hardness and Influence of Heat Treatment
4. Conclusions
- (1)
- By using the CALPHAD approach, the concentration of nickel in the experimental Al8Zn7Ni3Mg alloy has been justified. While the eutectic point in the Al-8%Zn-3%Mg-Ni system corresponds to 3.6%Ni, the 7%Ni composition is highly hypereutectic. Taking into account the opportunity to shift the solidification path with further refinement caused by increase in cooling rates, the experimental alloy comprises 12.8 vol.% Al3Ni intermetallics in which half corresponds to the primary phase;
- (2)
- Composition of the (Al) solid solution after rapid solidification has been simulated similar to the one at the 470 °C solid solution temperature. The higher the nickel content, the more saturated the (Al) matrix. It is shown that a 7%Ni concentration is advantageous in terms of obtaining supersaturated solid solution containing 9.6%Zn and 3.6%Mg, promoting precipitation of 9.6 vol.% of T’ and M’ dispersoids. Meanwhile, a higher amount of nickel does not provide a significant change in these values;
- (3)
- By OM, SEM, and TEM analysis, the increase in cooling rates on the microstructure was investigated, showing profound opportunities for microstructure tuning. A highly hypereutectic structure was observed after solidification at 0.1 K/s, 17 K/s, and 133 K/s accompanied with a refinement of the Al3Ni phase from 50 to 7 μm in medium size. A cooling rate of 1.4 × 103 K/s appeared to be sufficient for providing quasi-eutectic solidification manner, and most of the structure area is covered with fiber-like composite microstructure of 1.5 μm intermetallics, while the melt spinning provided a cooling rate of 2.3 × 105 K/s resulting in a visible hypoeutectic structure with ultrafine equiaxed 50 nm intermetallics in the (Al) matrix bulk, beneficial for looping reinforcing;
- (4)
- The hardness test revealed a substantial increase in strengthening as a result of structure refinement. While in slow and conventionally-cooled samples the hardness of 50–150 HV is relatively not contributed by Al3Ni intermetallics appearance, the rapidly solidified samples showed a significant enhancement of 165 HV in the 1 mm cast sample and 195 HV in melt-spun ribbons. Moreover, these samples both showed a significant strengthening after low temperature annealing at 200 °C, achieving up to 220 HV;
- (5)
- Nonetheless, a solid solution treatment at 470 °C resulted in significant degradation of hardness. While the 1 mm cast sample saw a decrease to the initial level, the melt-spun sample degraded to obtain hardness of around 140 HV. Such a loss in properties is caused by structure coarsening. Meanwhile, the fibrous-like intermetallics coalescenced to become needles and rods (up to 10 μm), and the globular particles evolved remaining a high roundness and relatively low size (0.2–2.5 μm), which is promising in terms of further consolidation processing.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Polmear, I.; StJohn, D.; Nie, J.-F.; Qian, M. Physical metallurgy of aluminium alloys. In Light Alloys, 5th ed.; Elseiver: London, UK, 2017; pp. 31–107. [Google Scholar] [CrossRef]
- Starke, E.A., Jr.; Staley, J.T. Application of modern aluminium alloys to aircraft. In Fundamentals of Aluminium Metallurgy; Lumley, R., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2011; pp. 747–783. [Google Scholar] [CrossRef]
- Ditta, A.; Weia, L.; Xub, Y.; Wua, S. Microstructural characteristics and properties of spray formed Zn-rich Al-Zn-Mg-Cu alloy under various aging conditions. Mater. Charact. 2020, 161, 110133. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Z.; Bai, S.; Zeng, D.; Luo, L.; Wang, J. Effects of natural aging on the formation and strengthening effect of G.P. zones in a retrogression and re-aged Al-Zn-Mg-Cu alloy. J. Alloy. Compd. 2020, 829, 154469. [Google Scholar] [CrossRef]
- Chen, Z.; Yuan, Z.; Ren, J. The mechanism of comprehensive properties enhancement in Al–Zn–Mg–Cu alloy via novel thermomechanical treatment. J. Alloy. Compd. 2020, 828, 154446. [Google Scholar] [CrossRef]
- Tamarin, Y. Atlas of Stress-Strain Curves, 2nd ed.; ASM International: Novelty, OH, USA, 2002. [Google Scholar]
- Peng, G.; Tietao, Z.; Xiaoqing, X.; Zhi, G.; Li, C. Refinement mechanism research of Al3Ni phase in Ni-7050 alloy. Rare Met. Mater. Eng. 2013, 42, 6–13. [Google Scholar] [CrossRef]
- Aoi, I.; Kuramoto, S.; Oh-ishi, K. Mechanical properties of Al-(8,10)%Zn-2%Mg-2%Cu base alloys processed with high-pressure torsion. Light Met. 2015, 179–182. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Samuel, A.M.; Alkahtani, S.A.; Samuel, F.H. A novel solution heat treatment of 7075-type alloy. Light Met. 2013, 383–390. [Google Scholar] [CrossRef]
- Wang, W.; Pana, Q.; Wanga, X.; Sun, Y.; Long, L.; Huang, Z. Mechanical properties and microstructure evolution of ultra-high strength Al-Zn-Mg-Cu alloy processed by room temperature ECAP with post aging. Mater. Sci. Eng. A 2018, 731, 195–208. [Google Scholar] [CrossRef]
- Elliot, R. Eutectic Solidification Processing: Crystalline and Glassy Alloys, 1st ed.; Butterworth-Heinemann: Oxford, UK, 1983. [Google Scholar] [CrossRef]
- Kim, C.S.; Cho, K.; Manjili, M.H.; Nezafati, M. Mechanical performance of particulate-reinforced Al metal-matrix composites (MMCs) and Al metalmatrix nano-composites (MMNCs). J. Mater. Sci. 2017, 52, 13319–13349. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, D.L. Contribution of Orowan strengthening effect in particulate-reinforced metal matrix nanocomposites. Mater. Sci. Eng. A 2008, 483–484, 148–152. [Google Scholar] [CrossRef]
- Garces, G.; Bruno, G.; Wanner, A. Load transfer in short fibre reinforced metal matrix composites. Acta Mater. 2007, 55, 5389–5400. [Google Scholar] [CrossRef]
- Garg, P.; Jamwal, A.; Kumar, D.; Sadasivuni, K.K.; Hussain, C.M.; Gupta, P. Advance research progresses in aluminium matrix composites: Manufacturing & applications. J. Mater. Res. Tech. 2019, 8, 4924–4939. [Google Scholar] [CrossRef]
- Chung, D.D.L. Metal-Matrix Composites. In Carbon Composites, 2nd ed.; Elseiver: London, UK, 2017; pp. 532–562. [Google Scholar] [CrossRef]
- Dinaharan, I. Liquid metallurgy processing of intermetallic matrix composites. In Intermetallic Matrix Composites; Mitra, R., Ed.; Elseiver: London, UK, 2017; pp. 167–202. [Google Scholar] [CrossRef]
- Gao, T.; Bian, Y.; Li, Z.; Xu, Q.; Yang, H.; Zhao, K.; Liu, X. Synthesis of a (ZrAl3 + AlN)/Al composite and the influence of particles content and element Cu on the microstructure and mechanical properties. J. Alloy. Compd. 2019, 791, 730–738. [Google Scholar] [CrossRef]
- Najarian, A.R.; Emadi, R.; Hamzeh, M. Fabrication of as-cast Al matrix composite reinforced by Al2O3/Al3Ni hybrid particles via in-situ reaction and evaluation of its mechanical properties. Mater. Sci. Eng. B 2018, 231, 57–65. [Google Scholar] [CrossRef]
- Bao, S.; Tang, K.; Kvithyld, A.; Engh, T.; Tangstad, M. Wetting of pure aluminium on graphite, SiC and Al2O3 in aluminium filtration. Trans. Nonferrous Met. Soc. China 2012, 22, 1930–1938. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, C.; Wang, R.; Peng, C.; Wu, X.; Li, H. Microstructure, mechanical and thermo-physical properties of Al–50Si–xMg alloys. Mater. Sci. Eng. A 2018, 730, 57–65. [Google Scholar] [CrossRef]
- Dobatkin, V.I.; Elagin, V.I.; Fedorov, V.M. Structure of rapidly solidified aluminium alloys. Adv. Perform. Mater. 1995, 2, 89–98. [Google Scholar] [CrossRef]
- Uzun, O.; Karaaslan, T.; Gogebakan, M.; Keskin, M. Hardness and microstructural characteristics of rapidly solidified Al–8–16 wt.%Si alloys. J. Alloy. Compd. 2004, 376, 149–157. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, C.; Wang, R.; Peng, C.; Qiu, K.; Feng, Y. Preparation of Al–Si alloys by a rapid solidification and powder metallurgy route. Mater. Des. 2015, 87, 996–1002. [Google Scholar] [CrossRef]
- Hatch, J.E. Aluminium: Properties and Physical Metallurgy; American Society for Metals: Cleveland, OH, USA, 1984. [Google Scholar]
- The Aluminium Association, International Alloy Designations and Chemical Composition Limits for Wrought Aluminium and Wrought Aluminium Alloys. The Aluminium Association. Available online: https://www.aluminum.org/sites/default/files/Teal%20Sheets.pdf (accessed on 26 April 2020).
- Fukui, Y.; Okada, H.; Kumazawa, N.; Watanabe, Y. Near-net-shape forming of Al-Al3Ni functionally graded material over eutectic melting temperature. Met. Mater. Trans. A 2000, 31, 2627–2636. [Google Scholar] [CrossRef]
- Nash, P.; Pan, Y.Y. The Al-Ni-Zr system (Aluminium-Nickel-Zirconium). J. Phase Equilibria 1991, 12, 105–113. [Google Scholar] [CrossRef]
- Belov, N.A.; Zolotorevskiy, V.S. The Effect of nickel on the structure, mechanical and casting properties of aluminium alloy of 7075 type. Mater. Sci. Forum 2002, 396–402, 935–940. [Google Scholar] [CrossRef]
- Belov, N.A.; Cheverikin, V.V.; Eskin, D.G.; Turchin, A.N. Effect of Al3Ni and Mg2Si eutectic phases on casting properties and hardening of an Al-7%Zn-3%Mg alloy. Mater. Sci. Forum 2006, 519–521, 413–418. [Google Scholar] [CrossRef]
- Wang, S.H.; Uan, J.Y.; Lui, T.S.; Chen, L.H. Examination on the aging and tensile properties of Al-Zn-Mg/Al3Ni eutectic composite. Met. Mater. Trans. A 2002, 33, 707–711. [Google Scholar] [CrossRef]
- Barclay, R.S.; Kerr, H.W.; Niessen, P. Off-eutectic composite solidification and properties in Al-Ni and Al-Co alloys. J. Mater. Sci. 1971, 6, 1168–1173. [Google Scholar] [CrossRef]
- Martínez-Villalobos, M.A.; Figueroa, I.A.; Suarez, M.A.; Rodríguez, G.A.L.; Peralta, O.N.; Reyes, G.G.; López, I.A.; Martínez, J.V.; Trujillo, C.D. Microstructural Evolution of Rapid Solidified Al-Ni Alloys. J. Mex. Chem. Soc. 2016, 60, 67–72. [Google Scholar] [CrossRef]
- Lin, Y.; Mao, S.; Yan, Z.; Zhang, Y.; Wang, L. The enhanced microhardness in a rapidly solidified Al alloy. Mater. Sci. Eng. A 2017, 692, 182–191. [Google Scholar] [CrossRef]
- Casati, R.; Coduri, M.; Riccio, M.; Rizzi, A.; Vedani, M. Development of a high strength Al–Zn–Si–Mg–Cu alloy for selective laser melting. J. Alloy. Compd. 2019, 801, 243–253. [Google Scholar] [CrossRef]
- Belov, N.A. Sparingly alloyed high-strength aluminium alloys: Principles of optimization of phase composition. Mater. Sci. Heat Tr. 2012, 53, 19–27. [Google Scholar] [CrossRef]
- Thermo-Calc Software TTAL5 Al-Alloys. Available online: http://www.thermocalc.com (accessed on 17 April 2020).
- Neikov, O.D.; Naboychenko, S.S.; Yefimov, N.A. Handbook of Non-Ferrous Metal Powders: Technologies and Applications, 2nd ed.; Elseiver: London, UK, 2018; p. 995. [Google Scholar] [CrossRef]
- Nishi, M.; Matsuda, K.; Miura, N.; Watanabe, K.; Ikeno, S.; Yoshida, T.; Murakami, S. Effect of the Zn/Mg ratio on microstructure and mechanical properties in Al-Zn-Mg alloys. Mater. Sci. Forum 2014, 794–796, 479–482. [Google Scholar] [CrossRef]
- Galy, C.; Le Guen, E.; Lacoste, E.; Arvieu, C. Main defects observed in aluminium alloy parts produced by SLM: From causes to consequences. Addit. Manuf. 2018, 22, 165–175. [Google Scholar] [CrossRef]
- Glazoff, M.; Khvan, A.; Zolotorevsky, V.; Belov, N.; Dinsdale, A. Casting Aluminium Alloys: Their physical and Mechanical Metallurgy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Grandfield, J.; Eskin, D.G.; Bainbridge, I. Direct-Chill Casting of Light Alloys: Science and Technology, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Todeschini, P.; Champier, G.; Samuel, F.H. Production of Al-(12–25) wt% Si alloys by rapid solidification: Melt spinning versus centrifugal atomization. J. Mater. Sci. 1992, 27, 3539–3551. [Google Scholar] [CrossRef]
- Ma, P.; Prashanth, K.G.; Scudino, S.; Jia, Y.; Wang, H.; Zou, C.; Wei, Z.; Eckert, J. Influence of Annealing on Mechanical Properties of Al-20Si Processed by Selective Laser Melting. Metals 2014, 4, 28–36. [Google Scholar] [CrossRef]
- Lekatou, A.; Sfikas, A.K.; Petsa, C.; Karantzalis, A.E. Al-Co alloys prepared by vacuum arc melting: Correlating microstructure evolution and aqueous corrosion behavior with Co content. Metals 2016, 6, 46. [Google Scholar] [CrossRef] [Green Version]









| Designation | Concentrations, wt. % | |||
|---|---|---|---|---|
| Zn | Mg | Ni | Al | |
| Al8Zn7Ni3Mg | 7.79 | 3.13 | 7.16 | Balance |
| Experimental Sample | Dendritic Parameter (d) 1, μm | Cooling Rate (Vc) 2, K/s | Al3Ni Size Range 1, μm | Al3Ni Median Size 1, μm (Al3NiE/Al3NiP) |
|---|---|---|---|---|
| FC | 198 ± 31 | 0.1 | 30–351 | 12/225 |
| 30 mm cast | 39 ± 6 | 17 | 20–94 | 4/13 |
| 5 mm cast | 19 ± 6 | 133 | 5–43 | 2/7 |
| 1 mm cast | 9 ± 2 | 1.4 × 103 | 1–3 | 1.5/- |
| MS | 1.6 ± 0.3 | 2.3 × 105 | 0.03–1.8 | 0.3/- |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shurkin, P.; Akopyan, T.; Korotkova, N.; Prosviryakov, A.; Bazlov, A.; Komissarov, A.; Moskovskikh, D. Microstructure and Hardness Evolution of Al8Zn7Ni3Mg Alloy after Casting at very Different Cooling Rates. Metals 2020, 10, 762. https://doi.org/10.3390/met10060762
Shurkin P, Akopyan T, Korotkova N, Prosviryakov A, Bazlov A, Komissarov A, Moskovskikh D. Microstructure and Hardness Evolution of Al8Zn7Ni3Mg Alloy after Casting at very Different Cooling Rates. Metals. 2020; 10(6):762. https://doi.org/10.3390/met10060762
Chicago/Turabian StyleShurkin, Pavel, Torgom Akopyan, Nataliya Korotkova, Alexey Prosviryakov, Andrey Bazlov, Alexander Komissarov, and Dmitry Moskovskikh. 2020. "Microstructure and Hardness Evolution of Al8Zn7Ni3Mg Alloy after Casting at very Different Cooling Rates" Metals 10, no. 6: 762. https://doi.org/10.3390/met10060762