Rescuing the Inhibitory Effect of the Salivary Gland Hypertrophy Virus of Musca domestica on Mating Behavior
Abstract
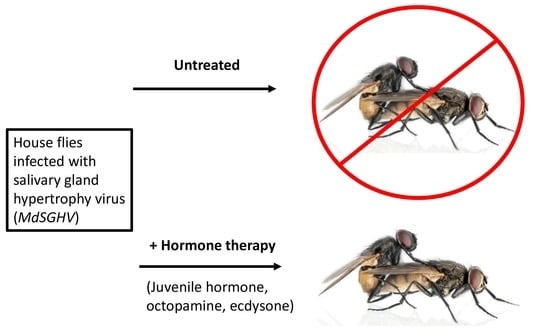
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Maintaining Flies
2.2. Infection with Virus
2.3. Hormone Treatments
2.4. Mating Timeline in House Flies
2.5. Observation of Mating Behavior
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Sluijs, L.; Liu, J.; Schrama, M.; van Hamond, S.; Vromans, S.P.J.M.; Scholten, M.H.; Žibrat, N.; Riksen, J.A.G.; Pijlman, G.P.; Sterken, M.G.; et al. Virus infection modulates male sexual behaviour in Caenorhabditis elegans. Mol. Ecol. 2021, 30, 6776–6790. [Google Scholar] [CrossRef] [PubMed]
- Burand, J.P.; Tan, W.; Kim, W.; Nojima, S.; Roelofs, W. Infection with the insect virus Hz-2v alters mating behavior and pheromone production in female Helicoverpa zea moths. J. Insect Sci. 2005, 5, 6. [Google Scholar] [CrossRef]
- Kariithi, H.M.; van Oers, M.M.; Vlak, J.M.; Vreysen, M.J.B.; Parker, A.G.; Abd-Alla, M.M.A. Virology, epidemiology and pathology of Glossina hytrosavirus, and its control prospects in laboratory colonies of the tsetse fly, Glossina pallidipes (Diptera; Glossinidae). Insects 2013, 4, 287–319. [Google Scholar] [CrossRef]
- Kariithi, H.; Meki, I.; Boucias, D.G.; Abd Alla, A. Hytrosaviruses: Current status and perspective. Curr. Opin. Ins. Sci. 2017, 22, 71–78. [Google Scholar] [CrossRef]
- Geden, C.J.; Lietze, V.-U.; Boucias, D.G. Seasonal prevalence and transmission of salivary gland hypertrophy virus of house flies (Diptera: Muscidae). J. Med. Entomol. 2008, 45, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Weeks, E.N.I.; Machtinger, E.T.; Geden, C.J.; Leemon, D.D. Biological control of livestock pests: Entomopathogens. In Ecology and Control of Vector-Borne Diseases; Garros, C., Bouyer, J., Takken, W., Smallegange, R.C., Eds.; Prevention and Control of Pests and Vector-borne Diseases in the Livestock Industry; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018; Volume 5, pp. 337–387. [Google Scholar]
- Mutika, G.N.; Marin, C.; Parker, A.G.; Vreysen, M.J.B.; Boucias, D.G. Abd-Alla AMM: Impact of salivary gland hypertrophy virus infection on the mating success of male Glossina pallidipes: Consequences for the sterile insect technique. PLoS ONE 2012, 7, e42188. [Google Scholar] [CrossRef]
- Coler, R.R.; Boucias, D.G.; Frank, J.H.; Maruniak, J.E.; Garcia Canedo, A.; Pendland, J.C. Characterization and description of a virus causing salivary gland hyperplasia in the housefly, Musca domestica. Med. Vet. Entomol. 1993, 7, 275–282. [Google Scholar] [CrossRef]
- Lietze, V.U.; Geden, C.J.; Blackburn, P.; Boucias, D.G. Effects of salivary gland hypertrophy virus on the reproductive behavior of the housefly, Musca domestica. Appl. Environ. Microbiol. 2007, 73, 6811–6818. [Google Scholar] [CrossRef]
- Kariithi, H.M.; Yao, X.; Yu, F.; Teal, P.E.; Verhoeven, C.P.; Boucias, D.G. Responses of the housefly, Musca domestica, to the hytrosavirus replication: Impacts on host’s vitellogenesis and immunity. Front. Microbiol. 2017, 8, 583. [Google Scholar] [CrossRef] [PubMed]
- Ringo, J.M. Sexual receptivity in insects. Annu. Rev. Entomol. 1996, 41, 473–494. [Google Scholar] [CrossRef]
- Teal, P.E.A.; Gomez-Simuta, Y.; Proveaux, A.T. Mating experience and juvenile hormone enhance sexual signaling and mating in male Caribbean fruit flies. Proc. Natl. Acad. Sci. USA 2000, 97, 3708–3712. [Google Scholar] [CrossRef] [PubMed]
- Tobin, E.N.; Stoffolano, J.G., Jr. The courtship of Musca species found in North America, 1. The housefly, Musca domestica. Ann. Entomol. Soc. Am. 1973, 66, 1249–1257. [Google Scholar] [CrossRef]
- Yin, C.-M.; Qin, W.-H.; Stoffolano, J.G., Jr. Regulation of mating behavior by nutrition and the corpus allatum in both male and female Phormia regina (Meigen). J. Insect Physiol. 1999, 45, 815–822. [Google Scholar] [CrossRef]
- Evans, B.P.; Stoffolano, J.G., Jr.; Yin, C.-M.; Meyer, J.S. A pharmacological and endocrinological study of female insemination in Phormia regina (Diptera: Calliphoridae). J. Insect Behav. 1997, 10, 493–508. [Google Scholar] [CrossRef]
- Evans, P.D. Biogenic amines in the insect nervous system. Adv. Insect Physiol. 1980, 15, 317–473. [Google Scholar]
- Schaler, J.; Stoffolano, J.G., Jr.; Fausto, A.M.; Gambellini, G.; Burand, J. Effect of diet on adult house fly (Diptera: Muscidae) injected with the salivary gland hypertrophy virus (MdSGHV). J. Insect Sci. 2018, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Sokal, R.R.; Rohlf, F.J. Biometry; W. H. Freeman: New York, NY, USA, 1980; 859p. [Google Scholar]
- Manning, A. Corpus allatum and sexual receptivity in female Drosophila melanogaster. Nature 1966, 211, 1321–1322. [Google Scholar] [CrossRef]
- Adams, T.S.; Hintz, A.M. Relationship of age, ovarian development, and the corpus allatum to mating in the housefly, Musca domestica. J. Insect Physiol. 1969, 15, 201–215. [Google Scholar] [CrossRef]
- Barth, R.H.; Lester, L.J. Neuro-hormonal control of sexual behavior in insects. Ann. Rev. Entomol. 1973, 18, 445–472. [Google Scholar] [CrossRef]
- Ringo, J.M. Hormonal regulation of sexual behavior in insects. Horm. Brain Behav. 2002, 3, 93–114. [Google Scholar] [CrossRef]
- Denlinger, D.L. Hormonal control of diapause. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon: Oxford, UK, 1985; Volume 8, pp. 353–412. [Google Scholar] [CrossRef]
- Stoffolano, J.G., Jr. Influence of diapause and diet on the development of the gonads and accessory reproductive glands of the black blowfly, Phormia regina (Meigen). Canandian J. Zool. 1974, 52, 981–988. [Google Scholar] [CrossRef]
- Tanigawa, N.A.; Shiga, S.; Numata, H. Role of the corpus allatum in the control of reproductive diapause in the male blow fly, Protophormia terraenovae. Zool. Sci. 1999, 16, 639–644. [Google Scholar] [CrossRef]
- Yin, C.-M.; Zou, B.-X.; Stoffolano, J.G., Jr. Precocene II treatment inhibits terminal oocyte development but not vitellogenin synthesis in the black blowfly Phormia regina. J. Insect Physiol. 1989, 35, 465–474. [Google Scholar] [CrossRef]
- Gruntenko, N.E.; Karpova, E.K.; Adonyeva, N.V.; Chentsova, N.A.; Faddeeva, N.V.; Alekseev, A.A.; Rauschenbach, I.Y. Juvenile hormone, 20-hydroxyecdysone and dopamine interaction in Drosophila virilis reproduction under normal and nutritional stress conditions. J. Insect Physiol. 2005, 51, 417–425. [Google Scholar] [CrossRef]
- Gruntenko, N.E.; Karpova, E.K.; Alekseev, A.A.; Chentsova, N.A.; Bogomolova, E.V.; Bownes, M.; Yu Rauschenbach, I. Effects of octopamine on reproduction, juvenile hormone metabolism, dopamine, and 20-hydroxyecdysone contents in Drosophila. Arch. Insect Biochem. Physiol. 2007, 65, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Rauschenbach, I.Y.; Chentsova, N.A.; Alekseev, A.A.; Gruntenko, N.E.; Karpova, E.K. Dopamine affects the level of 20-hydroxyecdysone in Drosophila virilis females. Dokl. Biol. Sci. 2006, 407, 179–181. [Google Scholar] [CrossRef]
- Gruntenko, N.E.; Rauschenbach, I.Y. Interplay of JH, 20E and biogenic amines under normal and stress conditions and its effect on reproduction. J. Insect Physiol. 2008, 54, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Barron, A.B.; Maleszka, J.; Vander Meer, R.K.; Robinson, G.R.; Maleszka, R. Comparing injection, feeding and topical application methods for treatment of honeybees with octopamine. J. Insect Physiol. 2007, 53, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Amsalem, E.; Teal, P.P.; Grozinger, C.M.; Hefetz, A. Precocene-I inhibits juvenile hormone biosynthesis, ovarian activation, aggression and alters sterility signal production in bumble bee (Bombus terrestris) workers. J. Exp. Biol. 2014, 217, 3178–3185. [Google Scholar] [CrossRef] [PubMed]
- Prompiboon, P.; Lietze, V.U.; Denton, J.S.; Geden, C.J.; Steenberg, T.; Boucias, D.G. Musca domestica salivary gland hypertrophy virus, a globally distributed insect virus that infects and sterilizes female houseflies. Appl. Environ. Microbiol. 2010, 76, 994–998. [Google Scholar] [CrossRef]
- Boucias, D.; Baniszewski, J.; Prompiboon, P.; Lietze, V.; Geden, C. Enhancement of the Musca domestica hytrosavirus infection with orally delivered reducing agents. J. Invert. Pathol. 2015, 124, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Geden, C.J.; Steenberg, T.; Lietze, V.U.; Boucias, D.G. Salivary gland hypertrophy virus of house flies in Denmark: Prevalence, host range, and comparison with a Florida isolate. J. Vector Ecol. 2011, 36, 231–238. [Google Scholar] [CrossRef]

| Treatment | Dose | # Mated/N b | %Mated c | Chi-Square d | |
|---|---|---|---|---|---|
| Uninfected | Infected | ||||
| vs | vs | ||||
| No injection/no treatment | N/A | 17/30 | 65.0 | - | 38.154 ** |
| PBS-injected control | 2.5 µL | 45/70 | 80.4 | 0.513 | 61.564 ** |
| Infected with MdSGHV | 2.5 µL | 0/45 | 0 | 33.010 ** | - |
| Inf. + Octopamine (OA) | 75 µg | 3/20 | 23.0 | 9.339 ** | 6.605 ** |
| Inf. + Methoprene (Meth) | 2 × 5 µg | 10/60 | 27.8 | 14.834 ** | 11.043 ** |
| Inf. + MdSGHV + acetone | 2.5 µL + 1 µL | 0/10 | 0 | 12.652 ** | 0.102 ns |
| Inf. + OA, Meth. + 20E | 5, 2.5 + 2.5 µg | 8/20 | 88.9 | 1.340 ns | 20.592 ** |
| Sugar-fed only | N/A | 0/10 | 0 | 12.652 ** | 0.102 ns |
| Sugar-fed only + Meth | 1 µL | 5/10 | 50.0 | 0.134 | 18.722 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallagher, M.; Ramirez, A.; Geden, C.J.; Stoffolano, J.G., Jr. Rescuing the Inhibitory Effect of the Salivary Gland Hypertrophy Virus of Musca domestica on Mating Behavior. Insects 2023, 14, 416. https://doi.org/10.3390/insects14050416
Gallagher M, Ramirez A, Geden CJ, Stoffolano JG Jr. Rescuing the Inhibitory Effect of the Salivary Gland Hypertrophy Virus of Musca domestica on Mating Behavior. Insects. 2023; 14(5):416. https://doi.org/10.3390/insects14050416
Chicago/Turabian StyleGallagher, Marissa, Arianna Ramirez, Christopher J. Geden, and John G. Stoffolano, Jr. 2023. "Rescuing the Inhibitory Effect of the Salivary Gland Hypertrophy Virus of Musca domestica on Mating Behavior" Insects 14, no. 5: 416. https://doi.org/10.3390/insects14050416