Varroa Appears to Drive Persistent Increases in New Zealand Colony Losses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials & Methods
2.1. Survey Design
2.2. Survey Enumeration
2.3. Survey Sample
2.4. Estimating Colony Losses and Confidence Intervals
3. Results
3.1. Colony Losses in New Zealand from 2016–2021
3.2. Beekeeping Operation Size and Colony Loss Levels
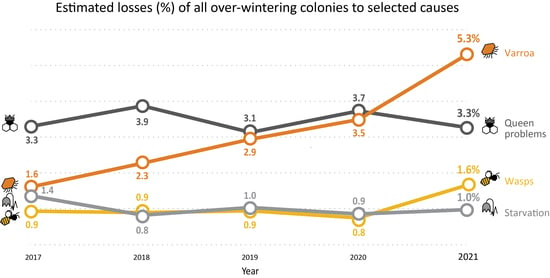
3.3. Factors Attributed to the Loss of Colonies during Winter
3.4. Varroa Mite Monitoring
3.5. Varroa Treatment in 2021
3.6. Loss Rates That Beekeepers Consider Acceptable
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gray, A.; Brodschneider, R.; Adjlane, N.; Ballis, A.; Brusbardis, V.; Charrière, J.-D.; Chlebo, R.; Coffey, F.M.; Cornelissen, B.; Amaro da Costa, C.; et al. Loss rates of honey bee colonies during winter 2017/18 in 36 countries participating in the COLOSS survey, including effects of forage sources. J. Apic. Res. 2019, 58, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Currie, R.W.; Pernal, S.F.; Guzmán-Novoa, E. Honey bee colony losses in Canada. J. Apic. Res. 2010, 49, 104–106. [Google Scholar] [CrossRef]
- Lee, K.V.; Steinhauer, N.; Rennich, K.; Wilson, M.E.; Tarpy, D.R.; Caron, D.M.; Rose, R.; Delaplane, K.S.; Baylis, K.; Lengerich, E.J.; et al. A national survey of managed honey bee 2013–2014 annual colony losses in the USA. Apidologie 2015, 46, 292–305. [Google Scholar] [CrossRef] [Green Version]
- Seitz, N.; Traynor, K.S.; Steinhauer, N.; Rennich, K.; Wilson, M.E.; Ellis, J.D.; Rose, R.; Tarpy, D.R.; Sagili, R.R.; Caron, D.M.; et al. A national survey of managed honey bee 2014–2015 annual colony losses in the USA. J. Apic. Res. 2015, 54, 292–304. [Google Scholar] [CrossRef]
- van der Zee, R.; Pisa, L.; Andonov, S.; Brodschneider, R.; Charrière, J.-D.; Chlebo, R.; Coffey, M.F.; Crailsheim, K.; Dahle, B.; Gajda, A.; et al. Managed honey bee colony losses in Canada, China, Europe, Israel and Turkey, for the winters of 2008–2009 and 2009–2010. J. Apic. Res. 2012, 51, 100–114. [Google Scholar] [CrossRef]
- Zee, R.v.d.; Brodschneider, R.; Brusbardis, V.; Charrière, J.-D.; Chlebo, R.; Coffey, M.F.; Dahle, B.; Drazic, M.M.; Kauko, L.; Kretavicius, J.; et al. Results of international standardised beekeeper surveys of colony losses for winter 2012–2013: Analysis of winter loss rates and mixed effects modelling of risk factors for winter loss. J. Apic. Res. 2014, 53, 19–34. [Google Scholar] [CrossRef] [Green Version]
- van der Zee, R.; Gray, A.; Pisa, L.; de Rijk, T. An Observational Study of Honey Bee Colony Winter Losses and Their Association with Varroa destructor, Neonicotinoids and Other Risk Factors. PLoS ONE 2015, 10, e0131611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodschneider, R.; Gray, A.; van der Zee, R.; Adjlane, N.; Brusbardis, V.; Charrière, J.-D.; Chlebo, R.; Coffey, M.F.; Crailsheim, K.; Dahle, B.; et al. Preliminary analysis of loss rates of honey bee colonies during winter 2015/16 from the COLOSS survey. J. Apic. Res. 2016, 55, 375–378. [Google Scholar] [CrossRef] [Green Version]
- Meixner, M.D.; Le Conte, Y. A current perspective on honey bee health. Apidologie 2016, 47, 273–275. [Google Scholar] [CrossRef] [Green Version]
- Neumann, P.; Carreck, N.L. Honey bee colony losses. J. Apic. Res. 2010, 49, 104–106. [Google Scholar] [CrossRef] [Green Version]
- Ausseil, A.E.; Dymond, J.R.; Newstrom, L. Mapping floral resources for honey bees in New Zealand at the catchment scale. Ecol. Appl. 2018, 28, 1182–1196. [Google Scholar] [CrossRef] [PubMed]
- Van Eaton, C. Manuka: The Biography of an Extraordinary Honey; Exisle Publishing: Auckland, New Zealand, 2014. [Google Scholar]
- Girma, A.; Seo, W.; She, R.C. Antibacterial activity of varying UMF-graded Manuka honeys. PLoS ONE 2019, 14, e0224495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, M.; McBride, M.; Dahiya, D.; Owusu-Apenten, R.; Nigam, P.S. Antibacterial activity of Manuka honey and its components: An overview. AIMS Microbiol. 2018, 4, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Ministry for Primary Industries, N.Z.; Situation Outlook for Primary Industries. December 2021. Available online: https://www.mpi.govt.nz/dmsdocument/49066-Situation-and-Outlook-for-Primary-Industries-SOPI-December-2021 (accessed on 5 April 2022).
- Newstrom-Lloyd, L.E. Pollination in New Zealand. In Ecosystem Services in New Zealand—Conditions and Trends; Dymond, J.R., Ed.; Manaaki Whenua Press: Lincoln, New Zealand, 2013; p. 412. [Google Scholar]
- Ministry for Primary Industries, New Zealand. 2021 Apiculture Monitoring Data. Available online: https://www.mpi.govt.nz/dmsdocument/48793-2021-Apiculture-monitoring-report-data (accessed on 5 April 2022).
- European Parliamentary Research Service. The EU’s Beekeeping Sector. Available online: https://epthinktank.eu/2017/10/24/the-eus-beekeeping-sector/ (accessed on 5 May 2022).
- Chauzat, M.P.; Cauquil, L.; Roy, L.; Franco, S.; Hendrikx, P.; Ribiere-Chabert, M. Demographics of the European apicultural industry. PLoS ONE 2013, 8, e79018. [Google Scholar] [CrossRef]
- Canadian Honey Council. Industry Overview—Canadian Apiculture Industry. Available online: https://honeycouncil.ca/industry-overview/ (accessed on 5 May 2022).
- Hall, R.J.; Pragert, H.; Phiri, B.J.; Fan, Q.-H.; Li, X.; Parnell, A.; Stanislawek, W.L.; McDonald, C.M.; Ha, H.J.; McDonald, W.; et al. Apicultural practice and disease prevalence in Apis mellifera, New Zealand: A longitudinal study. J. Apic. Res. 2021, 60, 644–658. [Google Scholar] [CrossRef]
- Brown, P.; Newstrom-Lloyd, L. Report on the 2015 New Zealand Colony Loss and Survival Study. In New Zealand Ministry for Primary Industries Technical Paper 2016/07; Ministry for Primary Industries: Wellington, New Zealand, 2016; p. 4. [Google Scholar]
- Brown, P. Report on the 2016 New Zealand Colony Loss Survey. In New Zealand Ministry for Primary Industries Technical Paper 2017/16; Ministry for Primary Industries: Wellington, New Zealand, 2017; p. 5. [Google Scholar]
- Brown, P. New Zealand Colony Loss Survey Report—2017. In New Zealand Ministry for Primary Industries Technical Paper 2018/10; Ministry for Primary Industries: Wellington, New Zealand, 2018; p. 3. [Google Scholar]
- Steinhauer, N.; Kulhanek, K.; Antúnez, K.; Human, H.; Chantawannakul, P.; Chauzat, M.-P.; van Engelsdorp, D. Drivers of colony losses. Curr. Opin. Insect Sci. 2018, 26, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Steinhauer, N.; Aureill, D.; Bruckner, S.; Wilson, M.; Rennich, K. United States Honey Bee Colony Losses 2020–2021: Preliminary Results Embargoed until Wednesday, 23 June 2021, 12.00 PM Noon CST. Available online: https://beeinformed.org/2021/06/21/united-states-honey-bee-colony-losses-2020-2021-preliminary-results/ (accessed on 13 May 2022).
- Stahlmann-Brown, P.; Robertson, T.; Borowik, O. Report on the 2020 New Zealand Colony Loss Survey. In New Zealand Ministry for Primary Industries Technical Paper 2021/04; Ministry for Primary Industries: Wellington, New Zealand, 2021; pp. 4–5. [Google Scholar]
- Stahlmann-Brown, P. New Zealand Colony Loss Survey Report—2021. In New Zealand Ministry for Primary Industries Technical Paper 2022/02; Ministry for Primary Industries: Wellington, New Zealand, 2022; p. 4. [Google Scholar]
- Kiesler, S.; Sproull, L.S. Response Effects in the Electronic Survey. Public Opin. Q. 1986, 50, 402–413. [Google Scholar] [CrossRef]
- Fowler, F.J. Survey Research Methods; SAGE Publications: Los Angeles, CA, USA, 2009. [Google Scholar]
- Crown Infrastructure Partners, New Zealand. Broadband Extended to 99.8% of the Population. Available online: https://www.crowninfrastructure.govt.nz/broadband-extended-to-99-8-of-the-population/ (accessed on 5 May 2022).
- van der Zee, R.; Gray, A.; Holzmann, C.; Pisa, L.; Brodschneider, R.; Chlebo, R.; Coffey, M.F.; Kence, A.; Kristiansen, P.; Mutinelli, F.; et al. Standard survey methods for estimating colony losses and explanatory risk factors in Apis mellifera. J. Apic. Res. 2013, 52, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Brodschneider, R.; Gray, A.; Adjlane, N.; Ballis, A.; Brusbardis, V.; Charrière, J.-D.; Chlebo, R.; Coffey, M.F.; Dahle, B.; de Graaf, D.C.; et al. Multi-country loss rates of honey bee colonies during winter 2016/2017 from the COLOSS survey. J. Apic. Res. 2018, 57, 452–457. [Google Scholar] [CrossRef] [Green Version]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models; CRC Press: Boca Raton, FL, USA, 1989; Volume 37. [Google Scholar]
- Van Engelsdorp, D.B.R.; Brostaux, Y.; van der Zee, R.; Pisa, L.; Underwood, R.; Lengerich, E.J.; Spleen, A.; Neumann, P.; Wilkins, S.; Budge, G.E.; et al. Calculating and Reporting Managed Honey Bee Colony Losses; CRC Press, Taylor Francis Group: Boca Raton, FL, USA, 2012. [Google Scholar]
- Azizi, H.R.; Sadeghi, E.; Taghdiri, M.; Vardanjani, A.R.K. The Comparative Evaluation of the Laboratory Methods of Separation Mite Varroa from the Mature Honeybee. Res. J. Parasitol. 2008, 3, 123–129. [Google Scholar] [CrossRef] [Green Version]
- De Jong, D.; De Andrea Roma, D.; Gonçalves, L.S. A comparative analysis of shaking solutions for the detection of Varroa jacobsoni on adult honey bees. Apidologie 1982, 13, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Fakhimzadeh, K. Potential of super-fine ground, plain white sugar dusting as an ecological tool for the control of Varroasis in the honey bee (Apis mellifera). Am. Bee J. 2000, 140, 487–491. [Google Scholar]
- Lee, K.; Reuter, G.; Spivak, M. Standardized sampling plan to detect varroa density in colonies and apiaries. Am. Bee J. 2010, 150, 1151–1155. [Google Scholar]
- Macedo, P.A.; Wu, J.; Ellis, M.D. Using inert dusts to detect and assess varroa infestations in honey bee colonies. J. Apic. Res. 2002, 41, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Brown, P.; Newstrom-Lloyd, L.E.; Foster, B.J.; Badger, P.H.; McLean, J.A. Winter 2016 honey bee colony losses in New Zealand. J. Apic. Res. 2018, 57, 278–291. [Google Scholar] [CrossRef] [Green Version]
- Brown, P.; Robertson, T. New Zealand Colony Loss Survey Report—2018, New Zealand Ministry for Primary Industries Technical Paper 2019/02; Ministry for Primary Industries: Wellington, New Zealand, 2019; p. 2.
- Oberreiter, H.; Brodschneider, R. Austrian COLOSS Survey of Honey Bee Colony Winter Losses 2018/19 and Analysis of Hive Management Practices. Diversity 2020, 12, 99. [Google Scholar] [CrossRef] [Green Version]
- Le Conte, Y.; Ellis, M.; Ritter, W. Varroa mites and honey bee health: Can Varroa explain part of the colony losses? Apidologie 2010, 41, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Millan-Leiva, A.; Marin, O.; Christmon, K.; van Engelsdorp, D.; Gonzalez-Cabrera, J. Mutations associated with pyrethroid resistance in Varroa mite, a parasite of honey bees, are widespread across the United States. Pest. Manag. Sci. 2021, 77, 3241–3249. [Google Scholar] [CrossRef]
- Rinkevich, F.D. Detection of amitraz resistance and reduced treatment efficacy in the Varroa Mite, Varroa destructor, within commercial beekeeping operations. PLoS ONE 2020, 15, e0227264. [Google Scholar] [CrossRef] [Green Version]
- Milani, N. The resistance of Varroa jacobsoni Oud. to acaricides. Apidologie 1999, 30, 229–234. [Google Scholar] [CrossRef]
- Colin, M.E.; Vandame, R.; Jourdam, P.; Di Pasquale, S. Fluvalinate resistance of Varroa jacobsoni Oudemans (Acari: Varroidae) in Mediterranean apiaries of France. Apidologie 1997, 28, 375–384. [Google Scholar] [CrossRef] [Green Version]
- Kulhanek, K.; Steinhauer, N.; Wilkes, J.; Wilson, M.; Spivak, M.; Sagili, R.R.; Tarpy, D.R.; McDermott, E.; Garavito, A.; Rennich, K.; et al. Survey-derived best management practices for backyard beekeepers improve colony health and reduce mortality. PLoS ONE 2021, 16, e0245490. [Google Scholar] [CrossRef] [PubMed]
- Mackay, J. What is happening with Varroa in New Zealand. Apiarist’s Advocate 2022, 2022, 22. [Google Scholar]
- Apiculture New Zealand. Colony Loss Survey reveals we need to do better with varroa. N. Z. Beekeep. 2022, 2022, 9–11. [Google Scholar]
- Taylor, M.; Goodwin, M. Control of Varroa: A Guide for New Zealand Beekeepers; Apiculture New Zealand: Wellington, New Zealand, 2021. [Google Scholar]
- Lester, P.J.; Felden, A.; Baty, J.W.; Bulgarella, M.; Haywood, J.; Mortensen, A.N.; Remnant, E.J.; Smeele, Z.E. Viral communities in the parasite Varroa destructor and in colonies of their honey bee host (Apis mellifera) in New Zealand. Sci. Rep. 2022, 12, 8809. [Google Scholar] [CrossRef]
- Loope, K.J.; Baty, J.W.; Lester, P.J.; Wilson Rankin, E.E. Pathogen shifts in a honeybee predator following the arrival of the Varroa mite. Proc. Biol. Sci. 2019, 286, 20182499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manley, R.; Temperton, B.; Boots, M.; Wilfert, L. Contrasting impacts of a novel specialist vector on multihost viral pathogen epidemiology in wild and managed bees. Mol. Ecol. 2020, 29, 380–393. [Google Scholar] [CrossRef]
- Donovan, B.J. Apoidea (Insecta: Hymenoptera). In Fauna of New Zealand; Manaaki Whenua Press: Lincoln, New Zealand, 2007; Volume 57, p. 295. [Google Scholar]
- Jacques, A.; Laurent, M.; Consortium, E.; Ribiere-Chabert, M.; Saussac, M.; Bougeard, S.; Budge, G.E.; Hendrikx, P.; Chauzat, M.P. A pan-European epidemiological study reveals honey bee colony survival depends on beekeeper education and disease control. PLoS ONE 2017, 12, e0172591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genersch, E.; von der Ohe, W.; Kaatz, H.; Schroeder, A.; Otten, C.; Büchler, R.; Berg, S.; Ritter, W.; Mühlen, W.; Gisder, S.; et al. The German bee monitoring project: A long term study to understand periodically high winter losses of honey bee colonies. Apidologie 2010, 41, 332–352. [Google Scholar] [CrossRef] [Green Version]
- Faurot-Daniels, C.; Glenny, W.; Daughenbaugh, K.F.; McMenamin, A.J.; Burkle, L.A.; Flenniken, M.L. Longitudinal monitoring of honey bee colonies reveals dynamic nature of virus abundance and indicates a negative impact of Lake Sinai virus 2 on colony health. PLoS ONE 2020, 15, e0237544. [Google Scholar] [CrossRef]







| Year | # of Respondents | % of All Registered Beekeepers | # of Colonies Reported | % of All Registered Colonies |
|---|---|---|---|---|
| 2021 | 4355 | 49.10% | 381,148 | 47.20% |
| 2020 | 2863 | 32.00% | 304,143 | 34.70% |
| 2019 | 3456 | 36.70% | 297,377 | 36.20% |
| 2018 | 3655 | 42.30% | 365,986 | 41.60% |
| 2017 | 2066 | 30.90% | 242,926 | 30.10% |
| 2016 | 2179 | 37.90% | 275,356 | 40.30% |
| 2015 | 366 | 6.70% | 225,660 | 39.60% |
| Year | Overall Loss Rate | 95% CI |
|---|---|---|
| 2021 | 13.59% | [13.21%, 13.99%] |
| 2020 | 11.30% | [10.95%, 11.66%] |
| 2019 | 10.41% | [10.05%, 10.77%] |
| 2018 | 10.20% | [9.85%, 10.57%] |
| 2017 | 9.70% | [9.37%, 10.05%] |
| 2016 | 9.53% | [9.07%, 10.02%] |
| 2015 | 8.37% | [7.66%, 9.15%] |
| Year | Queen Problems | Suspected Varroa | Suspected Starvation | Wasps | All Other Causes |
|---|---|---|---|---|---|
| 2021 | 24.0% | 38.9% | 7.0% | 12.0% | 18.1% |
| 2020 | 33.1% | 31.0% | 7.6% | 6.6% | 21.7% |
| 2019 | 30.3% | 28.1% | 10.3% | 9.6% | 21.7% |
| 2018 | 35.5% | 19.5% | 12.1% | 12.1% | 20.8% |
| 2017 | 34.4% | 16.9% | 13.9% | 9.7% | 25.1% |
| 2016 | 29.3% | see note * | 17.2% | 11.7% | 41.8% |
| 2015 | 35.7% | see note * | 15.6% | 14.9% | 33.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stahlmann-Brown, P.; Hall, R.J.; Pragert, H.; Robertson, T. Varroa Appears to Drive Persistent Increases in New Zealand Colony Losses. Insects 2022, 13, 589. https://doi.org/10.3390/insects13070589
Stahlmann-Brown P, Hall RJ, Pragert H, Robertson T. Varroa Appears to Drive Persistent Increases in New Zealand Colony Losses. Insects. 2022; 13(7):589. https://doi.org/10.3390/insects13070589
Chicago/Turabian StyleStahlmann-Brown, Philip, Richard J. Hall, Hayley Pragert, and Thomas Robertson. 2022. "Varroa Appears to Drive Persistent Increases in New Zealand Colony Losses" Insects 13, no. 7: 589. https://doi.org/10.3390/insects13070589