Forest Quality and Available Hostplant Abundance Limit the Canopy Butterfly of Teinopalpus aureus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Investigations of T. aureus and Magnoliaceae Trees
2.3. Calculation of the α Diversity Index and Determination of the Transect Length Unit
2.4. Environmental Data Collection
2.5. Hostplant Availability Definition
2.6. Statistical Analysis
3. Results
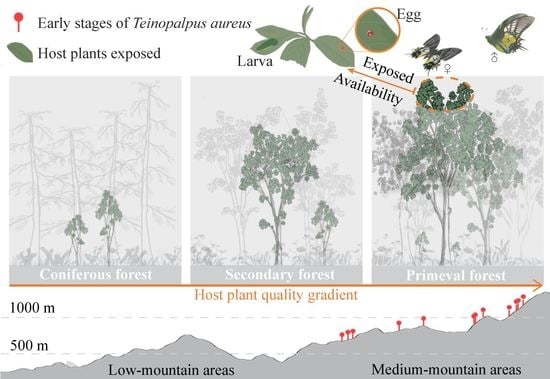
3.1. Distribution of T. aureus Driven by the Resource Quality of Hostplants
3.2. Habitat Preference and Requirements of T. aureus in Terms of Occurrence
3.3. Distribution and Drivers in T. aureus
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological Responses to Recent Climate Change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, O.; Settele, J.; Kudrna, O.; Klotz, S.; Kühn, I. Climate Change Can Cause Spatial Mismatch Of Trophlcally Interacting Species. Ecology 2008, 89, 3472–3479. [Google Scholar] [CrossRef]
- Schweiger, O.; Heikkinen, R.; Harpke, A.; Hickler, T.; Klotz, S.; Kudrna, O.; Kühn, I.; Pöyry, J.; Settele, J. Increasing Range Mismatching of Interacting Species under Global Change Is Related to Their Ecological Characteristics. Glob. Ecol. Biogeogr. 2012, 21, 88–99. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-Resolution Global Maps of 21st Century Forest Cover Change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef] [Green Version]
- Hanspach, J.; Schweiger, O.; Kühn, I.; Plattner, M.; Pearman, P.B.; Zimmermann, N.E.; Settele, J. Host Plant Availability Potentially Limits Butterfly Distributions under Cold Environmental Conditions. Ecography 2014, 37, 301–308. [Google Scholar] [CrossRef]
- Quinn, R.M.; Caston, K.J.; Roy, D.B. Coincidence in the Distributions of Butterflies and Their Foodplants. Ecography 1998, 21, 279–288. [Google Scholar] [CrossRef]
- Dennis, R.L.H.; Shreeve, T.G.; Arnold, H.R.; Roy, D.B. Does Diet Breadth Control Herbivorous Insect Distribution Size? Life History and Resource Outlets for Specialist Butterflies. J. Insect. Conserv. 2005, 9, 187–200. [Google Scholar] [CrossRef]
- Berzitis, E.; Minigan, J.; Hallett, R.; Newman, J. Climate and Host Plant Availability Impact the Future Distribution of the Bean Leaf Beetle (Cerotoma Trifurcata). Glob. Change Biol. 2014, 20, 2778–2792. [Google Scholar] [CrossRef] [PubMed]
- Curtis, R.J.; Brereton, T.M.; Dennis, R.L.H.; Carbone, C.; Isaac, N.J.B. Butterfly Abundance Is Determined by Food Availability and Is Mediated by Species Traits. J. Appl. Ecol. 2015, 52, 1676–1684. [Google Scholar] [CrossRef] [Green Version]
- Grillo, O.; Venora, G. Biodiversity Loss in a Changing Planet; InTech: Rijeka, Croatia, 2011; ISBN 978-953-307-707-9. [Google Scholar]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity Loss and Its Impact on Humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef]
- Karban, R.; Grof-Tisza, P.; Maron, J.L.; Holyoak, M. The Importance of Host Plant Limitation for Caterpillars of an Arctiid Moth (Platyprepia Virginalis) Varies Spatially. Ecology 2012, 93, 2216–2226. [Google Scholar] [CrossRef] [Green Version]
- Hutchinson, G.E. Concluding Remarks. Cold Spring Harb. Symp. Quant. Biol. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Matter, S.F.; Doyle, A.; Illerbrun, K.; Wheeler, J.; Roland, J. An Assessment of Direct and Indirect Effects of Climate Change for Populations of the Rocky Mountain Apollo Butterfly (Parnassius Smintheus Doubleday). Insect Sci. 2011, 18, 385–392. [Google Scholar] [CrossRef]
- Stenoien, C.; Nail, K.R.; Zalucki, J.M.; Parry, H.; Oberhauser, K.S.; Zalucki, M.P. Monarchs in Decline: A Collateral Landscape-Level Effect of Modern Agriculture. Insect Sci. 2018, 25, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.; Hill, J.K.; Thomas, J.; Asher, J.A.; Fox, R.; Huntley, B.; Roy, D.B.; Telfer, M.; Jeffcoate, S.H.P.; Harding, P.; et al. Rapid Responses of British Butterflies to Opposing Forces of Climate and Habitat Change. Nature 2001, 414, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Brereton, T.; Roy, D.; Greatorex-Davies, N. Thirty Years and Counting: The Contribution to Conservation and Ecology of Butterfly-Monitoring in the UK. Br. Wildl. 2006, 17, 162–170. [Google Scholar]
- Chowdhury, S.; Jennions, M.D.; Zalucki, M.P.; Maron, M.; Watson, J.E.M.; Fuller, R.A. Protected Areas and the Future of Insect Conservation. Trends Ecol. Evol. 2022, S0169-5347(22)00224-5. [Google Scholar] [CrossRef]
- Dennis, R.; Shreeve, T.; Van Dyck, H. Towards a Functional Resource-Based Concept for Habitat: A Butterfly Biology Viewpoint. Oikos 2003, 102, 417–426. [Google Scholar] [CrossRef]
- Dennis, R. Just How Important Are Structural Element as Habitat Components? Indications from a Declining Lycaenid with Priority Conservation Status. J. Insect Conserv. 2004, 8, 37–45. [Google Scholar] [CrossRef]
- Dennis, R.L.H. A Resource-Based Habitat View for Conservation: Butterflies in the British Landscape; John Wiley & Sons: Hoboken, NJ, USA, 2010; ISBN 978-1-4443-1526-4. [Google Scholar]
- Game, E.; Kareiva, P.; Possingham, H. Six Common Mistakes in Conservation Priority Setting. Conserv. Biol. J. Soc. Conserv. Biol. 2013, 27, 480–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spehn, E.M.; Rudmann-Maurer, K.; Korner, C.; Maselli, D. Mountain Biodiversity and Global Change; Global Mountain Biodiversity Assessment (GMBA) of DIVERSITAS: Basel, Switzerland, 2012. [Google Scholar]
- Zhou, Y. The Monograph of Chinese Butterflies; Henan Science and Technology Press: Zhengzhou, China, 1994. [Google Scholar]
- Li, X.; Zhang, Y.-L.; Settele, J.; Franzén, M.; Schweiger, O. Long-Distance Dispersal and Habitat Use of the Butterfly Byasa Impediens in a Fragmented Subtropical Forest. Insect Conserv. Divers. 2012, 6, 170–178. [Google Scholar] [CrossRef]
- Gao, K.; Li, X.; Guo, Z.; Zhang, Y. The Bionomics, Habitat Requirements and Population Threats of the Butterfly Bhutanitis Thaidina in Taibai Mountain. J. Insect Conserv. 2014, 18, 29–38. [Google Scholar] [CrossRef]
- Igarashi, S. Life history of Teinopalpus aureus in Vietnam in comparison with that of T. imperialis. Butterflies 2001, 30, 4–24. [Google Scholar]
- Wu, C.S.; Xu, Y.F. Butterflies of China; Straits Publishing House: Fuzhou, China, 2017. [Google Scholar]
- Zeng, J.P.; Zhou, S.Y.; Luo, B.T.; Qin, K.; Wu, J.S. Life History of Teinopalpus aureus guangxiensis Chou et Zhou (Lepidoptera: Papilionidae). Guangxi Sciences 2007, 14, 323–326. [Google Scholar]
- Zeng, J.P.; Zhou, S.Y.; Luo, B.T.; Qin, K.; Liang, Y.L. Morphology and bionomics of the endangered butterfly golden kaiserihind, Teinopalpus aureus, in Dayaoshan of Guangxi. Chin. Bull. Entomol. 2008, 45, 457–464. [Google Scholar]
- Lin, B.Z.; Zhu, X.F.; Zeng, J.P.; Yuan, J.X. Research on Biological Characteristics of Teinoplpus aureus in Jiulianshan. For. Res. 2017, 30, 399–408.49. [Google Scholar] [CrossRef]
- Xing, S.; Au, T.F.; Dufour, P.C.; Cheng, W.; Landry Yuan, F.; Jia, F.; Vu, L.V.; Wang, M.; Bonebrake, T.C. Conservation of Data Deficient Species under Multiple Threats: Lessons from an Iconic Tropical Butterfly (Teinopalpus Aureus). Biol. Conserv. 2019, 234, 154–164. [Google Scholar] [CrossRef]
- Wang, W.L.; Suman, D.; Zhang, H.H.; Xu, Z.-B.; Fang-Zhou, M.; Hu, S.J. Butterfly Conservation in China: From Science to Action. Insects 2020, 11, 661. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.M.; Morris, M.G. Threatened Swallowtail Butterflies of the World; IUCN: Gland, Switzerland, 1985; ISBN 978-2-88032-603-6. [Google Scholar]
- Gimenez The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/en (accessed on 3 September 2022).
- National Forestry and Grassland Administration (NFGA); Ministry of Agriculture and Rural Affairs (MARA). National Key Protected Wildlife List; Beijing, China. 2021. Available online: http://www.forestry.gov.cn/main/5461/20210205/122418860831352.html. (accessed on 13 June 2022).
- Zeng, J.P.; Zhou, S.Y.; Ding, J.; Luo, B.T.; Qin, K. Behavior characteristics and habitat adaptabilities of the endangered butterfly Teinopalpus aureus in Mount Dayao. Acta Ecol. Sin. 2012, 32, 6527–6534. [Google Scholar] [CrossRef]
- Igarashi, S. On the Life History of Teinopalpus Imperialis HOPE in Northern India and Its Phylogenetic Position in the Papilionidae. Lepid. Sci. 1987, 38, 115–151. [Google Scholar] [CrossRef]
- Zeng, J.P. Study on biological of Teinopalpus aureus Guangxi Chou et zhou. Master’s Thesis, Guangxi Normal University, Guilin, China, 2005. [Google Scholar]
- He, G.Q.; Jia, F.H. Research on population quantity and host plants of Teinoplpus aureus Mell in Jinggang Mountain. J. Nanchang Inst. Technol. 2012, 31, 68–70. [Google Scholar]
- Zeng, J.P.; Lin, B.Z.; Zhu, X.F.; Liu, L.Y. A Host Plant, Michelia maudiae, despread-istributed in South China for the Endangered Butterfly of Teinopalpus aureus. Acta Agric. Univ. Jiangxiensis 2014, 36, 550–555. [Google Scholar] [CrossRef]
- Liu, X.Z.; Xiao, Z.Y.; Ma, J.H. Scientific Survey and Study on the Forest Ecosystem in Jiangxi Jiulianshan Nature Reserve; China Forestry Publishing House: Beijing, China, 2009; pp. 287–311. [Google Scholar]
- Zeng, J.P.; Jin, Z.F.; Chen, F.S. Studies on Forest Ecology in Jiulianshan: Special Topics on Animals and Insects; Jiangxi Science and Technology Press: Nanchang, China, 2022. [Google Scholar]
- Ye, W.H.; Ma, K.P.; Ma, K.M.; Sang, W.G.; Gao, X.M. Studies on plant community diversity in Donglingshan mountain, Beijing, China IV. The influence of scale on α diversity. Acta Ecol. Sin. 1998, 18, 10–14. [Google Scholar]
- Roleček, J.; Vild, O.; Sladký, J.; Řepka, R. Habitat Requirements of Endangered Species in a Former Coppice of High Conservation Value. Folia Geobot. 2017, 52, 59–69. [Google Scholar] [CrossRef]
- Roberts, D.W.; Cooper, S.V. Concepts and Techniques of Vegetation Mapping. General technical report INT—U.S.; Department of Agriculture, Forest Service, Intermountain Research Station: Ogden, UT, USA, 1989. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/. (accessed on 5 September 2022).
- Ramírez-Fischer, F.J.; Benyamini, D.; Vargas, H.A. An Endangered Hemiparasitic Shrub Is the Only Host Plant of the Little-Known Neotropical Hairstreak Strymon Flavaria (Lepidoptera: Lycaenidae) in the Arid Andes. J. Insect Conserv. 2016, 20, 923–928. [Google Scholar] [CrossRef]
- Scherer, G.; Fartmann, T. Occurrence of an Endangered Grassland Butterfly Is Mainly Driven by Habitat Heterogeneity, Food Availability and Microclimate. Insect Sci. 2021, 29, 1211–1225. [Google Scholar] [CrossRef]
- Modin, H.; Öckinger, E. Mobility, Habitat Selection and Population Connectivity of the Butterfly Lycaena Helle in Central Sweden. J. Insect Conserv. 2020, 24, 821–831. [Google Scholar] [CrossRef]
- Paré, P.W.; Tumlinson, J.H. Plant Volatiles as a Defense against Insect Herbivores. Plant Physiol. 1999, 121, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadamiro, H.; Chen, L.; Akotsen-Mensah, C.; Setzer, W.N. Antennal Electrophysiological Responses of the Giant Swallowtail Butterfly, Papilio Cresphontes, to the Essential Oils of Zanthoxylum Clava-Herculis and Related Plants. Chemoecology 2010, 20, 25–33. [Google Scholar] [CrossRef]
- Damptey, F.G.; Adofo, E.; Duah-Gyamfi, A.; Adusu, D.; Opuni-Frimpong, E. Logging Effects on Seedling Regeneration and Diversity in a Tropical Moist Semi-Deciduous Forest in Ghana. Geol. Ecol. Landsc. 2021, 1–12. [Google Scholar] [CrossRef]
- Liu, Y.H. Magnolias of China; Beijing Science & Technology Press: Beijing, China, 2004; p. 6. [Google Scholar]
- Southwood, T.R.E. The Number of Species of Insect Associated with Various Trees. J. Anim. Ecol. 1961, 30, 1–8. [Google Scholar] [CrossRef]







| Environmental Variables | Abbreviations | Descriptions |
|---|---|---|
| Altitude | Altitude | the altitude of each transect section’s center point |
| Aspect | Aspect | the aspect of each transect section’s center point |
| Slope | Slope | the slope of each transect section’s center point |
| Average tree age | Age a | the average tree age in each transect section |
| Diameter of breast height | DBH b | the mean DBH of all Magnoliaceae trees in each transect section |
| Tree height | Height b | the mean tree height of all Magnoliaceae trees in each transect section |
| All Magnoliaceae species diversity | MSD b | the Shannon–Wiener index of all Magnoliaceae plants of each transect section |
| All Magnoliaceae plants abundance | MPAb b | the individual number of all Magnoliaceae plants of each transect section |
| All hostplants species diversity | HPSD b | the Shannon–Wiener index of all hostplants of each transect section |
| All hostplants abundance | HPAb b | the individual number of all hostplants of each transect section |
| Available hostplants species diversity | AHPSD b | the Shannon–Wiener index of the available hostplants with the height above the average tree height of each transect section |
| Available hostplants abundance | AHPAb b | the individual number of the available hostplants with the height above the average tree height of each transect section |
| Habitat type | Habitat | the habitat of each transect section, including primeval, secondary, or coniferous forest |
| Habitat Types | Actual Proportion Utilized (Pi) | Expected Proportion Utilized (Pio) | Bailey’s 95% Confidence Interval for Pi | Selection |
|---|---|---|---|---|
| Primeval forests | 0.8462 | 0.2101 | 0.4372 ≤ Pi ≤ 0.9847 | Preference |
| Secondary forests | 0.1538 | 0.6807 | 0.0021 ≤ Pi ≤ 0.4954 | Avoidance |
| Coniferous forests | 0 | 0.1092 | 0.0000 ≤ Pi ≤ 0.0000 | Avoidance |
| Environmental Variables | Non-Occupied (n = 106) | Occupied (n = 13) | p Value |
|---|---|---|---|
| Altitude | 662.68 ± 168.1 b | 983.00 ± 154.64 a | <0.001 *** |
| Aspect | 0.52 ± 0.31 a | 0.34 ± 0.25 a | >0.05 ns |
| Slope | 16.83 ± 8.78 b | 17.54 ± 6.09 a | >0.05 ns |
| Age | 46.46 ± 11.77 b | 60.15 ± 10.54 a | <0.001 *** |
| DBH | 8.43 ± 10.50 a | 17.51 ± 7.72 a | <0.01 ** |
| Height | 5.81 ± 6.91 b | 9.80 ± 3.11 a | <0.01 ** |
| MSD | 0.11 ± 0.27 b | 0.41 ± 0.37 a | <0.001 *** |
| MPAb | 6.08 ± 11.70 b | 24.23 ± 27.94 a | <0.001 *** |
| HPSD | 0.07 ± 0.22 b | 0.36 ± 0.32 a | <0.001 *** |
| HPAb | 3.35 ± 7.33 b | 23.85 ± 28.13 a | <0.001 *** |
| AHPSD | 0.04 ± 0.15 b | 0.29 ± 0.30 a | <0.001 *** |
| AHPAb | 1.46 ± 3.48 b | 15.15 ± 17.33 a | <0.001 *** |
| Environmental Variables | Standardized Discriminant Coefficient | Wilk’s λ | p |
|---|---|---|---|
| AHPAb | 1.464 | 0.613 | <0.001 |
| Altitude | 0.460 | 0.592 | <0.001 |
| Habitat | 0.334 | 0.572 | <0.001 |
| HPAb | −0.924 | 0.571 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wang, H.; Zha, Y.; Wei, H.; Chen, F.; Zeng, J. Forest Quality and Available Hostplant Abundance Limit the Canopy Butterfly of Teinopalpus aureus. Insects 2022, 13, 1082. https://doi.org/10.3390/insects13121082
Wang L, Wang H, Zha Y, Wei H, Chen F, Zeng J. Forest Quality and Available Hostplant Abundance Limit the Canopy Butterfly of Teinopalpus aureus. Insects. 2022; 13(12):1082. https://doi.org/10.3390/insects13121082
Chicago/Turabian StyleWang, Lu, Hui Wang, Yuhang Zha, Heyi Wei, Fusheng Chen, and Juping Zeng. 2022. "Forest Quality and Available Hostplant Abundance Limit the Canopy Butterfly of Teinopalpus aureus" Insects 13, no. 12: 1082. https://doi.org/10.3390/insects13121082