Habituation to a Deterrent Plant Alkaloid Develops Faster in the Specialist Herbivore Helicoverpa assulta Than in Its Generalist Congener Helicoverpa armigera and Coincides with Taste Neuron Desensitisation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Diets
2.3. Chemicals
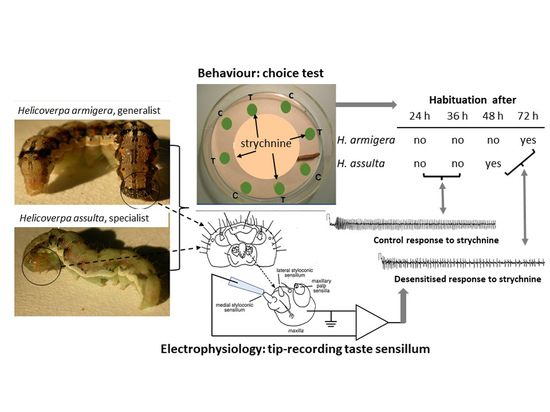
2.4. Experimental Design and Behavioral Bioassays
2.5. Electrophysiology
2.6. Statistical Analysis
3. Results
3.1. Behavioral Bioassays
3.2. Electrophysiological Responses
3.3. Relationship between Electrophysiological and Behavioral Responses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schoonhoven, L.M.; van Loon, J.J.A. An inventory of taste in caterpillars: Each species its own key. Acta. Zool. Acad. Sci. Hung. 2002, 48, 215–263. [Google Scholar]
- Zacharuk, R.Y.; Shields, V.D.C. Sensilla of immature insects. Annu. Rev. Entomol. 1991, 36, 331–354. [Google Scholar] [CrossRef]
- Bernays, E.A.; Chapman, R.F. The evolution of deterrent responses in plant-feeding insects. In Perspectives in Chemoreception and Behavior; Chapman, R.F., Bernays, E.A., Stoffolano, J.G., Jr., Eds.; Springer: New York, NY, USA, 1987; pp. 159–173. [Google Scholar]
- Bernays, E.A.; Oppenheim, S.; Chapman, R.F.; Kwon, H.; Gould, F. Taste sensitivity of insect herbivores to deterrents is greater in specialists than in generalists: A behavioral test of the hypothesis with two closely related caterpillars. J. Chem. Ecol. 2000, 26, 547–563. [Google Scholar] [CrossRef]
- Chapman, R.F.; Bernays, E.A. Insect behavior at the leaf surface and learning as aspects of host plant selection. Cell. Mol. Life Sci. 1989, 45, 215–222. [Google Scholar] [CrossRef]
- Dethier, V.G. The role of taste in food intake: A comparative view. In Mechanisms of Taste Transduction; Simon, S.A., Roper, S.D., Eds.; CRC: Boca Raton, FL, USA, 1993; pp. 3–25. [Google Scholar]
- Bernays, E.A.; Singer, M.S. Taste alteration and endoparasites. Nature 2005, 436, 476. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.P.; Renwick, J.A.A. Cross habituation to feeding deterrents and acceptance of a marginal host-plant by Pieris rapae larvae. Entomol. Exp. Appl. 1995, 76, 295–302. [Google Scholar] [CrossRef]
- Renwick, J.A.A.; Lopez, K. Experience-based food consumption by larvae of Pieris rapae: Addiction to glucosinolates? Entomol. Exp. Appl. 1999, 91, 51–58. [Google Scholar] [CrossRef]
- Zhou, D.S.; Wang, C.Z.; van Loon, J.J.A. Chemosensory basis of behavioural plasticity in response to deterrent plant chemicals in the larva of the small cabbage white butterfly Pieris rapae. J. Insect. Physiol. 2009, 55, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.S.; van Loon, J.J.A.; Wang, C.Z. Experience-based behavioral and chemosensory changes in the generalist insect herbivore Helicoverpa armigera exposed to two deterrent plant chemicals. J. Comp. Physiol. A. 2010, 196, 791–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, D.S.; Teng, T.; Liu, J.H.; Long, J.M. Cross-habituation to deterrents correlates with desensitisation of the corresponding deterrent neuron in the larva of the black cutworm, Agrotis ipsilon. Entomol. Exp. Appl. 2021, 169, 1039–1048. [Google Scholar] [CrossRef]
- Akhtar, Y.; Rankin, C.H.; Isman, M.B. Decreased response to feeding deterrents following prolonged exposure in the larvae of a generalist herbivore, Trichoplusia ni (Lepidoptera: Noctuidae). J. Insect. Behav. 2003, 16, 811–831. [Google Scholar] [CrossRef]
- Del Campo, M.L.; Miles, C.I.; Schroeder, F.C.; Müller, C.; Booker, R.; Renwick, J.A.A. Host recognition by the tobacco hornworm is mediated by a host plant compound. Nature 2001, 411, 186–189. [Google Scholar] [CrossRef]
- Miles, C.I.; Del Campo, M.L.; Renwick, J.A.A. Behavioral and chemosensory responses to a host recognition cue by larvae of Pieris rapae. J. Comp. Physiol. A. 2005, 191, 147–155. [Google Scholar] [CrossRef]
- van Loon, J.J.A.; Tang, Q.B.; Wang, H.L.; Wang, C.Z.; Zhou, D.S.; Smid, H.M. Tasting in plant-feeding insects: From single compounds to complex natural stimuli. In Insect Taste; Newland, P.L., Cobb, M., Marion-Poll, F., Eds.; Taylor and Francis: Abingdon, UK, 2008; pp. 103–126. [Google Scholar]
- Sollai, G.; Biolchini, M.; Crnjar, R. Taste receptor plasticity in relation to feeding history in two congeneric species of Papilionidae (Lepidoptera). J. Insect. Physiol. 2018, 107, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, M.L.; Miles, C.I. Chemosensory tuning to a host recognition cue in the facultative specialist larvae of the moth Manduca sexta. J. Exp. Biol. 2003, 206, 3979–3990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glendinning, J.I.; Davis, A.; Ramaswamy, S. Contribution of different taste cells and signaling pathways to the discrimination of “bitter” taste stimuli by an insect. J. Neurosci. 2002, 22, 7281–7287. [Google Scholar] [CrossRef]
- Glendinning, J.I.; Domdom, S.; Long, E. Selective adaptation to noxious foods by a herbivorous insect. J. Exp. Biol. 2001, 204, 3355–3367. [Google Scholar] [CrossRef] [PubMed]
- Glendinning, J.I.; Ensslen, S.; Eisenberg, M.E.; Weiskopf, P. Diet-induced plasticity in the taste system of an insect: Localization to a single transduction pathway in an identified taste cell. J. Exp. Biol. 1999, 202, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Glendinning, J.I.; Hills, T.T. Electrophysiological evidence for two transduction pathways within a bitter-sensitive taste receptor. J. Neurophysiol. 1997, 78, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Simmons, M.S.J.; Blaney, W.M. Some neurophysiological effects of azadirachtin on lepidopterous larvae and their feeding responses. In Proceedings of the Second International Neem Conference; Schmutterer, H., Ascher, K.R.S., Eds.; G.T.Z: Eschborn, Germany, 1983; pp. 163–180. [Google Scholar]
- van Loon, J.J.A. Chemoreception of phenolic acids and flavonoids in larvae of two species of Pieris. J. Comp. Physiol. A 1990, 166, 889–899. [Google Scholar] [CrossRef]
- Sollai, G.; Tomassini Barbarossa, I.; Masala, C.; Solari, P.; Crnjar, R. Gustatory sensitivity and food acceptance in two phylogenetically closely related papilionid species: Papilio hospiton and Papilio machaon. PLoS ONE 2014, 9, e100675. [Google Scholar]
- Fitt, G.P. The ecology of Heliothis species in relation to agroecosystems. Annu. Rev. Entomol. 1989, 34, 17–52. [Google Scholar] [CrossRef]
- Zhao, X.C.; Yan, Y.H.; Wang, C.Z. Behavioral and electrophysiological responses of Helicoverpa assulta, H. armigera (Lepidoptera: Noctuidae), their F1 hybrids and backcross progenies to sex pheromone component blends. J. Comp. Physiol. A 2006, 192, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.J.; Gong, P.Y. A new and practical artificial diet for the cotton bollworm. Entomol. Sin. 1997, 4, 277–282. [Google Scholar]
- Tang, Q.B.; Jiang, J.W.; Yan, Y.H.; Van Loon, J.J.A.; Wang, C.Z. Genetic analysis of larval host-plant preference in two sibling species of Helicoverpa. Entomol. Exp. Appl. 2006, 118, 221–228. [Google Scholar] [CrossRef]
- Wang, C.Z.; Dong, J.F.; Tang, D.L.; Zhang, J.H.; Li, W.; Qin, J. Host selection of Helicoverpa armigera and H. assulta and its inheritance. Prog. Nat. Sci. 2004, 14, 880–884. [Google Scholar] [CrossRef]
- Messchendorp, L.; van Loon, J.J.A.; Gols, G.J.Z. Behavioural and sensory responses to drimane antifeedants in Pieris brassicae larvae. Entomol. Exp. Appl. 1996, 79, 195–202. [Google Scholar] [CrossRef]
- Hodgson, E.S.; Lettvin, J.Y.; Roeder, K.D. Physiology of a primary receptor unit. Science 1955, 122, 417–418. [Google Scholar] [CrossRef] [PubMed]
- van Loon, J.J.A.; Schoonhoven, L.M. Specialist deterrent chemoreceptors enable Pieris caterpillars to discriminate between chemically different deterrents. Entomol. Exp. Appl. 1999, 91, 29–35. [Google Scholar] [CrossRef]
- Renwick, J.A.A.; Huang, X.P. Rejection of host-plant by larvae of cabbage butterfly: Diet-dependent sensitivity to an antifeedant. J. Chem. Ecol. 1995, 21, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Glendinning, J.I.; Brown, H.; Capoor, M.; Davis, A.; Gbedemah, A.; Long, E. A peripheral mechanism for behavioral adaptation to specific "bitter" taste stimuli in an insect. J. Neurosci. 2001, 21, 3688–3696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jermy, T.; Hanson, F.E.; Dethier, V.G. Induction of specific food preference in lepidopterous larvae. Entomol. Exp. Appl. 1967, 41, 211–230. [Google Scholar]
- Schoonhoven, L.M. Loss of host plant specificity by Manduca sexta after rearing on an artificial diet. Entomol. Exp. Appl. 1967, 10, 270–272. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Zhou, D.S.; Gao, S.X.; Zhao, X.C.; Tang, Q.B.; Wang, C.Z.; van Loon, J.J.A. Higher plasticity in feeding preference of a generalist than a specialist: Experiments with two closely related Helicoverpa species. Sci. Rep. 2017, 7, 17876. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.L.; Shields, V.D. An electrophysiological analysis of the effect of phagostimulant mixtures on the responses of a deterrent-sensitive cell of gypsy moth larvae, Lymantria dispar (L.). Arthropod-Plant Int. 2012, 6, 259–267. [Google Scholar] [CrossRef]
- Hiroi, M.; Meunier, N.; Marion-Poll, F.; Tanimura, T. Two antagonistic gustatory receptor neurons responding to sweet-salty and bitter taste in Drosophila. J. Neurobiol. 2004, 61, 333–342. [Google Scholar] [CrossRef]
- Bernays, E.A.; Chapman, R.F. A neurophysiological study of sensitivity to a feeding deterrent in two sister species of Heliothis with different diet breadths. J. Insect. Physiol. 2000, 46, 905–912. [Google Scholar] [CrossRef]
- Inagaki, H.K.; de-Leon, S.B.T.; Wong, A.M.; Jagadish, S.; Ishimoto, H.; Barnea, G.; Kitamoto, T.; Axel, R.; Anderson, D.J. Visualizing neuromodulation in vivo: TANGO-mapping of dopamine signaling reveals appetite control of sugar sensing. Cell 2012, 148, 583–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.L.; Hou, W.H.; Zhang, J.J.; Dang, Y.L.; Yang, Q.Y.; Zhao, X.C.; Ma, Y.; Tang, Q.B. Plant metabolites drive different responses in caterpillars of two closely related Helicoverpa species. Front. Physiol. 2021, 12, 460. [Google Scholar] [CrossRef]
- Szentesi, A.; Bernays, E.A. A study of behavioural habituation to a feeding deterrent in nymphs of Schistocerca gregaria. Physiol. Entomol. 1984, 9, 329–340. [Google Scholar] [CrossRef]
- French, A.; Ali Agha, M.; Mitra, A.; Yanagawa, A.; Sellier, M.J.; Marion-Poll, F. Drosophila bitter taste (s). Front. Integr. Neurosci. 2015, 9, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.V.; Raghuwanshi, R.P.; Shen, W.L.; Montell, C. Food experience-induced taste desensitization modulated by the Drosophila TRPL channel. Nature Neurosci. 2013, 16, 1468–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glendinning, J.I.; Gonzalez, N.A. Gustatory habituation to deterrent allelochemicals in a herbivore—concentration and compound specificity. Anim. Behav. 1995, 50, 915–927. [Google Scholar] [CrossRef]
- Akhtar, Y.; Isman, M.B. Generalization of a habituated feeding deterrent response to unrelated antifeedants following prolonged exposure in a generalist herbivore, Trichoplusia ni. J. Chem. Ecol. 2004, 30, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Glendinning, J.I.; Valcic, S.; Timmermann, B.N. Maxillary palps can mediate taste rejection of plant allelochemicals by caterpillars. J. Comp. Physiol. A 1998, 183, 35–43. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.-S.; Wang, C.-Z.; van Loon, J.J.A. Habituation to a Deterrent Plant Alkaloid Develops Faster in the Specialist Herbivore Helicoverpa assulta Than in Its Generalist Congener Helicoverpa armigera and Coincides with Taste Neuron Desensitisation. Insects 2022, 13, 21. https://doi.org/10.3390/insects13010021
Zhou D-S, Wang C-Z, van Loon JJA. Habituation to a Deterrent Plant Alkaloid Develops Faster in the Specialist Herbivore Helicoverpa assulta Than in Its Generalist Congener Helicoverpa armigera and Coincides with Taste Neuron Desensitisation. Insects. 2022; 13(1):21. https://doi.org/10.3390/insects13010021
Chicago/Turabian StyleZhou, Dong-Sheng, Chen-Zhu Wang, and Joop J. A. van Loon. 2022. "Habituation to a Deterrent Plant Alkaloid Develops Faster in the Specialist Herbivore Helicoverpa assulta Than in Its Generalist Congener Helicoverpa armigera and Coincides with Taste Neuron Desensitisation" Insects 13, no. 1: 21. https://doi.org/10.3390/insects13010021