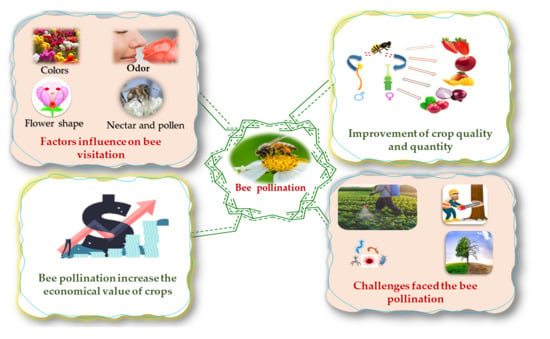
Overview of Bee Pollination and Its Economic Value for Crop Production
Abstract
:Simple Summary
Abstract
1. Introduction
2. Effect of Bee Pollination on the Economy
3. Role of Bee Pollination in Crop Production (Quality and Quantity)
3.1. Honey Bees
3.2. Bumble Bees
3.3. Stingless Bees
3.4. Carpenter Bees
3.5. Solitary Bees
| Crop (Species) | Bee Pollinator | Impact on Crop Yield | Country | Reference |
|---|---|---|---|---|
| Fruits | ||||
| Apple (Malus domestica L.) | Honey bees (Apis mellifera L.) | Enhancing fruit production with high yield and quality (fruit size and number of seeds). | Pakistan | [91] |
| Wild bees and honeybees (A. mellifera) | Seed number increased with bee abundance which consequently increased fruit quality. | China | [92] | |
| Stingless bees (Melipona quadrifasciata anthidioides Lepeletier) Africanised honeybee (A. mellifera) | Both stingless bees (12 hives/hectare) and Africanized honeybees (7 hives/ hectare) provided higher seed and fruit production than supplementation with honeybees alone. | Brazil | [93] | |
| Honey bee (A. mellifera) | Increased fruit set by 15%, seed set and content of fruit sugar, and farmer’s profits by 70%. | Argentina | [54] | |
| Bumble bees (B. impatiens) and honey bee (A. mellifera) | The quantity and quality of fruits produced from pollination from both species were equivalent. | Canada | [66] | |
| Wild bees | Fruit set increased | USA | [94] | |
| Coconut (Cocos nucifera L.) | Honey bees (A. mellifera) | Increased fruit set | Mexico | [48] |
| Honey bees (A. mellifera) | Effective pollinators compared to wasp | Jamaica | [95] | |
| Watermelon (Citrullus lanatus Thunb.) | Honey bees (A. mellifera) | Fruit set, fruit numbers and weights per plot increased linearly as number of honey bees visits increased. | USA | [96] |
| Tart cherry (Prunus cerasus L.) | Osmia lignaria solitary bee | Cherry weight increased by 2.8% compared to the control. | Utah | [97] |
| Cape gooseberry (Physalis peruviana L.) | Honey bees (A. mellifera) | Improvement of fruit mass by 30.3%, equatorial diameter by 13.3%, seed variety by 7%, and seed mass by 8.4%. | Colombia | [55] |
| Sweet cherry (Prunus avium L.) | Wild bees and honey bees | Fruit set was enhanced compared to open pollination. | Germany | [98] |
| Almond (Prunus dulcis (Mill.) D.A.Webb) | Honey bees (A. mellifera) | Increased fruit set by 60% and kernel yield by 20% compared to self-pollination. | USA | [29] |
| Solitary Bee (O. cornuta) | Increased fruit production was parallel with increased visits by O. cornuta. | Spain | [99] | |
| Avocado (Persea americana Mill.) | Honey bees (A. mellifera) | High pollination efficiency for fruit set, increased the production, and improved the weight of the fruit. | In Central America | [100] |
| Passion fruit (Passiflora edulis Sims. f. flavicarpa Deg) | Honey bees (A. mellifera), and carpenter bees (Xylocopa spp.) | The diversity of bee species affected the fruit set and fruit quality and led to a higher reproductive efficiency. | Australia and Philippins | [82,83] |
| Native Brazilian bees (Xylocopa spp.) | Production costs lowered by 58%. Average production was 7000 kg/hectare/year. | Brazil | [101] | |
| Citrus (Citrus sinensis L.) | Honey bees (A. mellifera) | Lead to heavier fruit with less acid content and fewer seeds per bud. | Brazil | [102] |
| Mango (Mangifera indica L.) | Honey bees (A. cerana) | Fruit setting was 42.29% compared to open pollination 33.36%. | India | [103] |
| Guava (Psidium guajava L.) | Honey bees (A. mellifera) | Increased fruit set; improved the quality of fruit length and girth. | India | [47] |
| Strawberry (Fragaria × ananassa DUCH) | Osmia bicornis L. | Increased commercial value by 54.3% compared with self-pollination and by 38.6% compared with wind pollination. Number of fertilized achenes increased, and improved post-harvest quality occurred (more intensive red colour and lower sugar acid ratios). | Germany | [104] |
| Bees | Quantity and quality improved. Yield increased 20%. | Germany | [50] | |
| European orchard bee (Osmia cornuta Latr) | Fruit weight was higher than the control treatment. | Germany | [105] | |
| Kiwifruit (Actinidia Deliciosa) | Honey bees (A. mellifera) | Increased fruit set and yield. | Australia | [106] |
| Bumble bee (Bombus haemorrhoidalis Smith) | Higher fruit breadth, longer fruits, heavier fruits, higher healthy fruits, and higher fruit set. | India | [61] | |
| Pear (Pyrus communis L.) | Honey bees (A. mellifera) | Fruit size increased by 7% and lead to USD 400 per hectare net increase in income. | USA | [46] |
| Cranberries (Vaccinium oxycoccos L.) | Honey bees (A. mellifera) | Production increased from 3.7 million in 1989 to 5.4 million in 1998. | USA | [45] |
| Vegetables | ||||
| Cucumbers (Cucumis sativus L.) | Honey bees (A. mellifera) | 10% increase in production. | USA | [45] |
| Stingless bee (Heterotrigona itama) | Lead to larger, heavier, and longer cucumbers. | Terengganu | [73] | |
| Sweet pepper (Capsicum annuum L.) | Bumble bee (Bombus impatiens Cr.) | Increased fruit weight, width, and volume. Increased seed weight and reduced harvesting time. | Canada | [62] |
| Bumble bees (Bombus terrestris L.) | Increased yields, fruit weight, and quality of seed, and fruits under unheated greenhouse conditions. Seed set was 49.8% compared to 27.5% of the control (self-pollination) treatment. | Spain | [63] | |
| Tomatoes (Solanum lycopersicum L.) | Bumble bee (Anthophora urbana Cresson and Bombus vosnesenskii Radoszkowski) | Lead to higher yield and improved the quality of fruits. | USA | [107] |
| Bees (Exomalopsis analis Spinola, Centris tarsata Smith, Bombus morio Swederus, Eulaema nigrita Lepeletier and Epicharis sp.) | Increased fruit production and quality. | Brazil | [108] | |
| Aromatic and medicinal plants | ||||
| Anise (Pimpinella anisum L.) | Honey bees (A. mellifera) | Increasing seed yield/feddan to 781.55 kg compared to 300.24 Kg for control group (insect exclusion). | Egypt | [12] |
| Black Seed (Nigella sativa L.) | Honeybee (A. mellifera) | Increased yield and seed setting but no effect on the weight of the seed produced. | Pakistan | [109] |
| Cumin (Cuminum cyminum L.) | Apis florea F., A. mellifera and A. dorsata | Enhanced yield by 40.03% compared to 41.37% for open pollination. | India | [11] |
| Sunflowers (Helianthus annuus L.) | Wild bees and honey bees (A. mellifera) | Interactions between wild and honey bees increased the efficiency of pollination up to 5-fold compared to honey bees only. | USA | [21] |
| Africanized honey bees (A. mellifera) | The average yield of seeds was 43% higher compared to the control. | Brazil | [13] | |
| Honey bees (Apis mellifera L.) | Played a significant role in pollination compared to moths and wind. | Central Darling Downs | [110] | |
| Coriander (Coriandrum sativum Linnaeus.) | Apis cerana Fabricius | The seed set was significantly higher by 69.51% compared to 54.89% in the control group. The yield was 14.57 q/hectare vs 11.66 q/hectare in the control group. | India | [14] |
| Other plants | ||||
| Cotton Gossypium hirsutum L.) | Honey bees (A. mellifera) | Increased production by more than 12% for fiber weight and over 17% for seed number. | Brazil | [111] |
| Honeybees and wild bees | Significantly increased yield quantity and quality by an average of 62%. The average yield was 953.91 kg/hectare. | West Africa | [32] | |
| Pumpkins (Cucurbita maxima L.) | Honey bees (A. mellifera) | Fruit set, fruit size, weight, and number of seeds increased linearly with the number of visits. | Brazil | [112] |
| Soyabean (Glycine max L.) | Honey bees (A. mellifera) | Yield increase was associated with an increase of the seed number. | Argentina | [113] |
| Honey bees (A. mellifera) | Increased yield by 18.09%. | Brazil | [114] | |
| Sesame (Sesamum indicum L.) | Honeybees (A. mellifera) and wild bees | The mean yield of seed was 202.20 kg/hectare. The exclusion of pollinators caused an average yield gap of 59%. | West Africa | [32] |
| C. canephora L | Apis dorsata F. | Bees increased fruit production of coffee by 50% more than wind. | South India | [115] |
| Cowpea (Vigna unguiculata L. Walp) | Honey bees and bumble bees | NR | Nigeria | [116] |
| Red clover seed (Trifolium pratense L.) legume | Bumble bee (B. vosnesenskii) | High yield and most production of seeds. | USA | [19] |
| Pineland golden trumpet (Angadenia berteroi (A.DC.) Miers) | Long-tongued bee (Megachile georgica Cresson and Melissodes communis communis) | NR | USA | [117] |
| Mustard (Brassica juncea L.) | Honey bees (A. mellifera) | Increased fruit set, viability of seed, seed yield, and oil nutrient contents in the seed. | India | [118] |
| Honey bees (A. cerana) | Increased siliqua/panicle by 20.8%, seeds/silique by 9.4%, and seed yield by 17.1% compared to open pollination. | India | [119] | |
| Green grams (Vigna radiate L.) and Bambara groundnut (Voandzeia subterranean L.) | Feral bees | Enhanced yield and improved the quality of crops. | Kenya | [33] |
| Coffee (Coffea arabica L.) | Solitary bees and social bees | Significantly increased fruit set. | Indonesia | [120] |
| Acai palm (Euterpe oleracea Martius) | Stingless bee (Scaptotrigona aff. postica) | Increased the production reach to 2.5 times. The increase was evident as per the number of fruits per bunch and fruit size. | Brazil | [121] |
| Oilseed rape (Brassica napus L.) | Solitary mason bee (Osmia rufa L.) | Increased fruit set, yield, and the number of seeds per pod by bee density. | Germany | [122] |
| Honey bees (A. mellifera) | Increased oil and decreasing chlorophyll content. | Sweden | [50] | |
| Honey bees (A. mellifera), and wild bees (Lasioglossum spp.). | Average yield was increased up to 37.5%. | France | [123] | |
4. Bee Visitation
5. Challenges Faced in Bee Pollination
6. Bee Pollination vs. Non-Bee Pollination
6.1. Hoverflies vs. Bees
6.2. Butterflies vs. Bees
6.3. Moths vs. Bees
6.4. Beetles vs. Bees
6.5. Thrips vs. Bees
6.6. Wasps vs. Bees
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gill, R.J.; Baldock, K.C.; Brown, M.J.; Cresswell, J.E.; Dicks, L.V.; Fountain, M.T.; Garratt, M.P.; Gough, L.A.; Heard, M.S.; Holland, J.M.O.J. Protecting an ecosystem service: Approaches to understanding and mitigating threats to wild insect pollinators. Adv. Ecol. Res. 2016, 54, 135–206. [Google Scholar] [CrossRef] [Green Version]
- Breeze, T.D.; Bailey, A.P.; Balcombe, K.G.; Potts, S.G. Pollination services in the UK: How important are honeybees? Agric. Ecosyst. Environ. 2011, 142, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Hristov, P.; Neov, B.; Shumkova, R.; Palova, N. Significance of apoidea as main pollinators. Ecological and economic impact and implications for human nutrition. Diversity 2020, 12, 280. [Google Scholar] [CrossRef]
- Sukumaran, A.; Khanduri, V.P.; Sharma, C.M. Pollinator-mediated self-pollination and reproductive assurance in an isolated tree of Magnolia grandiflora L. Ecol. Process. 2020, 9, 45–53. [Google Scholar] [CrossRef]
- García-Breijo, F.; Armiñana, J.R.; Garmendia, A.; Cebrián, N.; Beltrán, R.; Merle, H. In vivo pollen tube growth and evidence of self-pollination and prefloral anthesis in cv. Macabeo (Vitis vinifera L.). Agriculture 2020, 10, 647. [Google Scholar] [CrossRef]
- MacInnis, G.; Forrest, J.R.K. Field design can affect cross-pollination and crop yield in strawberry (Fragaria x ananassa D.). Agric. Ecosyst. Environ. 2020, 289, 106738–106745. [Google Scholar] [CrossRef]
- Lobo, J.A.; Quesada, M.; Stoner, K.E. Effects of pollination by bats on the mating system of Ceiba pentandra (Bombacaceae) populations in two tropical life zones in Costa Rica. Am. J. Bot. 2005, 92, 370–376. [Google Scholar] [CrossRef]
- Zeng, X.; Fischer, G.A. Wind pollination over 70 years reduces the negative genetic effects of severe forest fragmentation in the tropical oak Quercus bambusifolia. Heredity (Edinb). 2020, 124, 156–169. [Google Scholar] [CrossRef]
- Van der Kooi, C.J.; Ollerton, J. The origins of flowering plants and pollinators. Science (80-.) 2020, 368, 1306–1308. [Google Scholar] [CrossRef]
- Gallai, N.; Salles, J.M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Meena, N.K.; Lal, G.; Meena, R.S.; Meena, B.M.; Meena, R.D. Pollinator’s diversity and abundance on cumin (Cuminum cyminum L.) and their impact on yield enhancement at semi-arid regions. J. Entomol. Zool. Stud. 2018, 6, 1017–1021. [Google Scholar]
- Abd El-Wahab, T.E.; Ebadah, I.M.A.; Mahmoud, Y.A. Insect pollinators of anise plants (Pimpinella anisum L.) and the important role of honey bees (Apis mellifera L.) on their yield productivity. Arch. Phytopathol. Plant Prot. 2012, 45, 677–685. [Google Scholar] [CrossRef]
- Chambó, E.D.; Garcia, R.C.; de Oliveira, N.T.E.; Duarte-Júnior, J.B. Honey bee visitation to sunflower: Effects on pollination and plant genotype. Sci. Agric. 2011, 68, 647–651. [Google Scholar] [CrossRef]
- Patil, P.N.; Pastagia, J.J. Effect of bee pollination on yield of coriander. Coriandrum sativum Linnaeus. Int. J. Plant Prot. 2016, 9, 79–83. [Google Scholar]
- Klein, A.M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderone, N.W. Insect pollinated crops, insect pollinators and US agriculture: Trend analysis of aggregate data for the period 1992–2009. PLoS ONE 2012, 7, e37235. [Google Scholar] [CrossRef] [Green Version]
- Rader, R.; Bartomeus, I.; Garibaldi, L.A.; Garratt, M.P.D.; Howlett, B.G.; Winfree, R.; Cunningham, S.A.; Mayfield, M.M.; Arthur, A.D.; Andersson, G.K.S.; et al. Non-bee insects are important contributors to global crop pollination. Proc. Natl. Acad. Sci. USA 2016, 113, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Stanley, D.A.; Msweli, S.M.; Johnson, S.D. Native honeybees as flower visitors and pollinators in wild plant communities in a biodiversity hotspot. Ecosphere 2020, 11, e02957. [Google Scholar] [CrossRef]
- Rao, S.; Stephen, W.P. Bumble bee pollinators in red clover seed production. Crop Sci. 2009, 49, 2207–2214. [Google Scholar] [CrossRef]
- Bänsch, S.; Tscharntke, T.; Gabriel, D.; Westphal, C. Crop pollination services: Complementary resource use by social vs solitary bees facing crops with contrasting flower supply. J. Appl. Ecol. 2021, 58, 476–485. [Google Scholar] [CrossRef]
- Greenleaf, S.S.; Kremen, C. Wild bees enhance honey bees’ pollination of hybrid sunflower. Proc. Natl. Acad. Sci. USA 2006, 103, 13890–13895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannini, T.C.; Cordeiro, G.D.; Freitas, B.M.; Saraiva, A.M.; Imperatriz-Fonseca, V.L. The dependence of crops for pollinators and the economic value of pollination in Brazil. J. Econ. Entomol. 2015, 108, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Lautenbach, S.; Seppelt, R.; Liebscher, J.; Dormann, C.F. Spatial and temporal trends of global pollination benefit. PLoS One 2012, 7, e35954. [Google Scholar] [CrossRef] [Green Version]
- Aizen, M.A.; Garibaldi, L.A.; Cunningham, S.A.; Klein, A.M. How much does agriculture depend on pollinators? Lessons from long-term trends in crop production. Ann. Bot. 2009, 103, 1579–1588. [Google Scholar] [CrossRef]
- Montoya, D.; Gaba, S.; de Mazancourt, C.; Bretagnolle, V.; Loreau, M. Reconciling biodiversity conservation, food production and farmers’ demand in agricultural landscapes. Ecol. Modell. 2020, 416, 108889–108909. [Google Scholar] [CrossRef]
- Geeraert, L.; Aerts, R.; Berecha, G.; Daba, G.; De Fruyt, N.; D’hollander, J.; Helsen, K.; Stynen, H.; Honnay, O. Effects of landscape composition on bee communities and coffee pollination in Coffea arabica production forests in southwestern Ethiopia. Agric. Ecosyst. Environ. 2020, 288, 106706–106717. [Google Scholar] [CrossRef]
- Luo, D.; Silva, D.P.; De Marco Júnior, P.; Pimenta, M.; Caldas, M.M. Model approaches to estimate spatial distribution of bee species richness and soybean production in the Brazilian Cerrado during 2000 to 2015. Sci. Total Environ. 2020, 737, 139674. [Google Scholar] [CrossRef]
- Sáez, A.; Aizen, M.A.; Medici, S.; Viel, M.; Villalobos, E.; Negri, P. Bees increase crop yield in an alleged pollinator-independent almond variety. Sci. Rep. 2020, 10, 3177–3183. [Google Scholar] [CrossRef] [Green Version]
- Losey, J.E.; Vaughan, M. The economic value of ecological services provided by insects. Bioscience 2006, 56, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Esquivel, I.L.; Coulson, R.N.; Brewer, M.J. A native bee, melissodes tepaneca (Hymenoptera: Apidae), benefits cotton production. Insects 2020, 11, 487. [Google Scholar] [CrossRef]
- Stein, K.; Coulibaly, D.; Stenchly, K.; Goetze, D.; Porembski, S.; Lindner, A.; Konaté, S.; Linsenmair, E.K. Bee pollination increases yield quantity and quality of cash crops in Burkina Faso, West Africa. Sci. Rep. 2017, 7, 17691–17700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasina, J.M.; Mburu, J.; Kraemer, M.; Holm-Mueller, K. Economic benefit of crop pollination by bees: A case of kakamega small-holder farming in Western Kenya. J. Econ. Entomol. 2009, 102, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Hipólito, J.; Sousa, B.d.S.B.; Borges, R.C.; de Brito, R.M.; Jaffé, R.; Dias, S.; Imperatriz Fonseca, V.L.; Giannini, T.C. Valuing nature’s contribution to people: The pollination services provided by two protected areas in Brazil. Glob. Ecol. Conserv. 2019, 20, e00782. [Google Scholar] [CrossRef]
- Orges, R.C.B.; Rito, R.M.B.; Onseca, V.L.I.M.; Iannini, T.C.G. The value of crop production and pollination services in the Eastern Amazon. Neotrop. Entomol. 2020, 49, 545–556. [Google Scholar] [CrossRef]
- Reilly, J.R.; Artz, D.R.; Biddinger, D.; Bobiwash, K.; Boyle, N.K.; Brittain, C.; Brokaw, J.; Campbell, J.W.; Daniels, J.; Elle, E.; et al. Crop production in the USA is frequently limited by a lack of pollinators. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200922–20200930. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.A.; Jones, J.; Rocchetti, M.; Wright, D.; Rader, R. Bee visitation and fruit quality in berries under protected cropping vary along the length of polytunnels. J. Econ. Entomol. 2020, 113, 1337–1346. [Google Scholar] [CrossRef]
- Valido, A.; Rodríguez-Rodríguez, M.C.; Jordano, P. Honeybees disrupt the structure and functionality of plant-pollinator networks. Sci. Rep. 2019, 9, 4711–4721. [Google Scholar] [CrossRef] [Green Version]
- Garantonakis, N.; Varikou, K.; Birouraki, A.; Edwards, M.; Kalliakaki, V.; Andrinopoulos, F. Comparing the pollination services of honey bees and wild bees in a watermelon field. Sci. Hortic. (Amsterdam) 2016, 204, 138–144. [Google Scholar] [CrossRef]
- Hung, K.-L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172140–20172147. [Google Scholar] [CrossRef] [Green Version]
- Streinzer, M.; Brockmann, A.; Nagaraja, N.; Spaethe, J. Sex and caste-specific variation in compound eye norphology of five honeybee species. PLoS ONE 2013, 8, e57702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, D.; Choi, W.T.; Lin, H.; Qu, Z.; Breedveld, V.; Meredith, J.C. Humidity-tolerant rate-dependent capillary viscous adhesion of bee-collected pollen fluids. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Jeon, H.; Jung, C. Foraging behaviour and preference of pollen sources by honey bee (Apis mellifera) relative to protein contents. J. Ecol. Environ. 2020, 44, 4–10. [Google Scholar] [CrossRef]
- Seo, H.J.; Song, J.; Yoon, H.J.; Lee, K.Y. Effects of nectar contents on the foraging activity of honeybee (Apis mellifera) on Asian pear (Pyrus pyrifolia Nakai). Sci. Hortic. (Amsterdam) 2019, 245, 185–192. [Google Scholar] [CrossRef]
- Morse, R.A. and Calderone, N.W. The value of honey bees as pollinators of U.S. crops in 2000. Bee Cult. 2000, 128, 1–15. [Google Scholar] [CrossRef]
- Naumann, K.; Winston, M.L.; Slessor, K.N.; Smirle, M.J. Synthetic honey bee (Hymenoptera: Apidae) queen mandibular gland pheromone applications affect pear and sweet cherry pollination. J. Econ. Entomol. 1994, 87, 1595–1599. [Google Scholar] [CrossRef]
- Rajagopal, D.; Eswarappa, G.; Raju, A.J.S. Pollination Potentiality of Honeybees in Increasing Productivity of Guava in Karnataka. In Changing Trends in Pollen Spore Research; Today and Tomorrow’s Printers & Publishers: New Delhi, India, 2005; pp. 131–141. ISBN 8173715173. [Google Scholar]
- Meléndez-Ramírez, V.; Parra-Tabla, V.; Kevan, P.G.; Ramírez-Morillo, I.; Harries, H.; Fernández-Barrera, M.; Zizumbo-Villareal, D. Mixed mating strategies and pollination by insects and wind in coconut palm (Cocos nucifera L. (Arecaceae)): Importance in production and selection. Agric. For. Entomol. 2004, 6, 155–163. [Google Scholar] [CrossRef]
- Ewies, M.A.; EL-Sahhar, K.F. Observations on the behaviour of honeybees on onion and their effects on seed yield. J. Apic. Res. 1977, 16, 194–196. [Google Scholar] [CrossRef]
- Bartomeus, I.; Potts, S.G.; Steffan-Dewenter, I.; Vaissière, B.E.; Woyciechowski, M.; Krewenka, K.M.; Tscheulin, T.; Roberts, S.P.M.; Szentgyörgyi, H.; Westphal, C.; et al. Contribution of insect pollinators to crop yield and quality varies with agricultural intensification. PeerJ 2014, 2014, e328–e347. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Wahab, T.E.; Ebadah, I.M.A. Impact of honeybee and other insect pollinators on the seed setting and yield production of black cumin Nigella sativa L. J. Basic. Appl. Sci. Res 2011, 1, 622–626. [Google Scholar]
- Saboor, N.; Muhammad, A.; Muhammad, A.R.; Younisand, A. Role of pollinators in recommended and densely grown black cumin (Nigella sativa L.) yield at Dera Ismail Khan. J. Entomol. Zool. Stud. 2018, 6, 983–986. [Google Scholar]
- Bendifallah, L.; Louadi, K.; Doumandji, S. Bee fauna potential visitors of coriander flowers Coriandrum sativum L. (Apiaceae) in the Mitidja area (Algeria). J. Apic. Sci. 2013, 57, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Geslin, B.; Aizen, M.A.; Garcia, N.; Pereira, A.; Vaissière, B.E. The impact of honey bee colony quality on crop yield and farmers’ profit in apples and pears. Agric. Ecosyst. Environ. 2017, 248, 153–161. [Google Scholar] [CrossRef]
- Chautá-mellizo, A.; Campbell, S.A.; Argenis, M.; Thaler, J.S.; Poveda, K. Effects of natural and artificial pollination on fruit and offspring quality. Basic Appl. Ecol. 2012, 13, 524–532. [Google Scholar] [CrossRef]
- Isaacs, R.; Kirk, A.K. Pollination services provided to small and large highbush blueberry fields by wild and managed bees. J. Appl. Ecol. 2010, 47, 841–849. [Google Scholar] [CrossRef]
- Crowther, L.P.; Wright, D.J.; Richardson, D.S.; Carvell, C.; Bourke, A.F.G. Spatial ecology of a range-expanding bumble bee pollinator. Ecol. Evol. 2019, 9, 986–997. [Google Scholar] [CrossRef] [Green Version]
- Velthuis, H.H.W.; Van Doorn, A. A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 2006, 37, 421–451. [Google Scholar] [CrossRef] [Green Version]
- Peat, J.; Darvill, B.; Ellis, J.; Goulson, D. Effects of climate on intra- and interspecific size variation in bumble-bees. Funct. Ecol. 2005, 19, 145–151. [Google Scholar] [CrossRef]
- Fijen, T.P.M.; Scheper, J.A.; Boom, T.M.; Janssen, N.; Raemakers, I.; Kleijn, D. Insect pollination is at least as important for marketable crop yield as plant quality in a seed crop. Ecol. Lett. 2018, 21, 1704–1713. [Google Scholar] [CrossRef]
- Nayak, R.K.; Rana, K.; Sharma, H.K.; Rana, V.S.; Thakur, M. Influence of bumble bee pollination on quantitative and qualitative parameters of kiwifruit. Indian J. Hortic. 2019, 76, 294–299. [Google Scholar] [CrossRef]
- Shipp, J.L.; Whitfield, G.H.; Papadopoulos, A.P. Effectiveness of the bumble bee, Bombus impatiens Cr.(Hymenoptera: Apidae), as a pollinator of greenhouse sweet pepper. Sci. Hortic. (Amsterdam) 1994, 57, 29–39. [Google Scholar] [CrossRef]
- Roldán Serrano, A.; Guerra-Sanz, J.M. Quality fruit improvement in sweet pepper culture by bumblebee pollination. Sci. Hortic. (Amsterdam) 2006, 110, 160–166. [Google Scholar] [CrossRef]
- Desjardins, È.C.; De Oliveira, D. Commercial bumble bee Bombus impatiens (Hymenoptera: Apidae) as a pollinator in lowbush blueberry (Ericale: Ericaceae) fields. J. Econ. Entomol. 2006, 99, 443–449. [Google Scholar] [CrossRef]
- Yankit, P.; Rana, K.; Kumar Sharma, H.; Thakur, M.; Thakur, R.K. Effect of bumble bee pollination on quality and yield of Tomato (Solanum lycopersicum Mill.) grown under protected conditions. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Normandeau Bonneau, M.; Samson-Robert, O.; Fournier, V.; Chouinard, G. Commercial bumble bee (Bombus impatiens) hives under exclusion netting systems for apple pollination in orchards. Renew. Agric. Food Syst. 2020, 36, 234–244. [Google Scholar] [CrossRef]
- Quezada-Euán, J.J.G. Stingless Bees of Mexico. In Stingless Bees of Mexico: The Biology, Management and Conservation of an Ancient Heritage; Springer: New York, NY, USA, 2018; pp. 1–37. ISBN 9783319777849. [Google Scholar]
- Ramírez, V.M.; Ayala, R.; González, H.D. Crop pollination by stingless bees. In Pot-Pollen in Stingless Bee Melittology; Springer: Cham, Switzerland, 2018; pp. 139–153. [Google Scholar]
- Eickwort, G.C.; Ginsberg, H.S. Foraging and mating behavior in Apoidea. Annu. Rev. Entomol. 1980, 25, 421–446. [Google Scholar] [CrossRef]
- De Luca, P.A.; Vallejo-Marín, M. What’s the “buzz” about? The ecology and evolutionary significance of buzz-pollination. Curr. Opin. Plant Biol. 2013, 16, 429–435. [Google Scholar] [CrossRef]
- Del Sarto, M.C.L.; Peruquetti, R.C.; Campos, L.A.O. Evaluation of the neotropical stingless bee Melipona quadrifasciata (Hymenoptera: Apidae) as pollinator of greenhouse tomatoes. J. Econ. Entomol. 2005, 98, 260–266. [Google Scholar] [CrossRef]
- Solange, A.B.S.; Ana, C.R.; Luci, R.B. Pollination of Cucumber Cucumis sativus L. (Cucurbitales: Cucurbitaceae), by the stingless bees Scaptotrigona aff. depili Moure and Nannotrigona testaceicornis Lepeletier (Hymenoptera: Meliponini) in greenhouses. Neotrop. Entomol. 2008, 37, 506–512. [Google Scholar]
- Azmi, W.A.; Ghazi, R.; Sultan, U.; Abidin, Z.; Chuah, T.; Mara, U.T. Effects of stingless bee (Heterotrigona itama) pollination on greenhouse cucumber (Cucumis sativus). Malaysian Appl. Biol. 2017, 46, 51–55. [Google Scholar]
- Azmi, W.A.; Sembok, W.Z.W.; Yusuf, N.; Hatta, M.F.M.; Salleh, A.F.; Hamzah, M.A.H.; Ramli, S.N. Effects of pollination by the indo-Malaya stingless bee (Hymenoptera: Apidae) on the quality of greenhouse- produced rockmelon. J. Econ. Entomol. 2019, 112, 20–24. [Google Scholar] [CrossRef]
- Roselino, A.C.; Santos, S.B.; Hrncir, M.; Bego, L.R. Differences between the quality of strawberries (Fragaria x ananassa) pollinated by the stingless bees Scaptotrigona aff. depilis and Nannotrigona testaceicornis. Genet. Mol. Res. 2009, 8, 539–545. [Google Scholar] [CrossRef]
- Ilva, P.N.U.; Rncir, M.H.; Inês, C.; Ilva, S. Stingless bees, Melipona fasciculata, as efficient pollinators of eggplant (Solanum melongena) in greenhouses. Apidology 2013, 44, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Leys, R.; Cooper, S.J.B.; Schwarz, M.P. Molecular phylogeny of the large carpenter bees, genus Xylocopa (Hymenoptera: Apidae), based on mitochondrial DNA sequences. Mol. Phylogenet. Evol. 2000, 17, 407–418. [Google Scholar] [CrossRef]
- Somanathan, H.; Saryan, P.; Balamurali, G.S. Foraging strategies and physiological adaptations in large carpenter bees. J. Comp. Physiol. A Neuroethol. Sensory, Neural, Behav. Physiol. 2019, 205, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Keasar, T. Large carpenter bees as agricultural pollinators. Psyche A J. Entomol. 2010, 2010, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Richards, M.H. Built environments influence carpenter bee sociality and vice versa. Insectes Soc. 2020, 67, 201–202. [Google Scholar] [CrossRef]
- Raju, A.J.S.; Rao, S.P. Nesting habits, floral resources and foraging ecology of large carpenter bees (Xylocopa latipes and Xylocopa pubescens) in India. Curr. Sci. 2006, 1210–1217. [Google Scholar]
- Halder, S.; Ghosh, S.; Khan, R.; Khan, A.A.; Perween, T.; Hasan, M.A. Role of pollination in fruit crops: A review. Pharma Innov. J. 2019, 8, 695–705. [Google Scholar]
- Barrera, W.B., Jr.; Trinidad, K.A.D.; Presas, J.A. Hand pollination and natural pollination by carpenter bees (Xylocopa spp.) in Passiflora edulis Sims. f. flavicarpa Deg. (yellow passion fruit). J. Apic. Res. 2020. [Google Scholar] [CrossRef]
- Silveira, M.V.; Abot, A.R.; Nascimento, J.N.; Rodrigues, E.T. Is manual pollination of yellow passion fruit completely dispensable? Sci. Hortic. (Amsterdam) 2012, 146, 99–103. [Google Scholar] [CrossRef]
- Hogendoorn, K.; Steen, Z.; Schwarz, M.P. Native Australian carpenter bees as a potential alternative to introducing bumble bees for tomato pollination in greenhouses. J. Apic. Res. 2000, 39, 67–74. [Google Scholar] [CrossRef]
- Adi, S.; Avi, S.; Tamar, K. The carpenter bee Xylocopa pubescens as an agricultural pollinator in greenhouses. Apidologie 2007, 38, 508–517. [Google Scholar]
- Batra, S.W. Solitary bees. Sci. Am. 1984, 250, 120–127. [Google Scholar] [CrossRef]
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Kleijn, D.; Winfree, R.; Bartomeus, I.; Carvalheiro, L.G.; Henry, M.; Isaacs, R.; Klein, A.M.; Kremen, C.; M’Gonigle, L.K.; Rader, R.; et al. Delivery of crop pollination services is an insufficient argument for wild pollinator conservation. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Garratt, M.P.D.; Breeze, T.D.; Boreux, V.; Fountain, M.T.; McKerchar, M.; Webber, S.M.; Coston, D.J.; Jenner, N.; Dean, R.; Westbury, D.B.; et al. Apple pollination: Demand depends on variety and supply depends on pollinator identity. PLoS ONE 2016, 11, e0153889. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.R.; Khan, M.R. The role of honey bees Apis mellifera L. (Hymenoptera: Apidae) in pollination of apple. Pakistan J. Biol. Sci. 2004, 7, 359–362. [Google Scholar]
- Wu, P.; Tscharntke, T.; Westphal, C.; Wang, M.; Olhnuud, A.; Xu, H.; Yu, Z.; van der Werf, W.; Liu, Y. Bee abundance and soil nitrogen availability interactively modulate apple quality and quantity in intensive agricultural landscapes of China. Agric. Ecosyst. Environ. 2021, 305, 107168–107176. [Google Scholar] [CrossRef]
- Viana, B.F.; da Encarnacao Coutinho, J.G.; Garibaldi, L.A.; Braganca Castagnino, G.L.; Gramacho, K.P.; Oliveira Silva, F. Stingless bees further improve apple pollination and production. J. Pollinat. Ecol. 2014, 14, 261–269. [Google Scholar] [CrossRef]
- Mallinger, R.E.; Gratton, C. Species richness of wild bees, but not the use of managed honeybees, increases fruit set of a pollinator-dependent crop. J. Appl. Ecol. 2015, 52, 323–330. [Google Scholar] [CrossRef]
- Free, J.B.; Raw, A.; Williams, I.H. Pollination of coconut (Cocos nucifera L.) in Jamaica by honeybees and wasps. Appl. Anim. Ethol. 1975, 1, 213–223. [Google Scholar] [CrossRef]
- Walters, S.A. Honey bee pollination requirements for triploid watermelon. HortScience 2005, 40, 1268–1270. [Google Scholar] [CrossRef] [Green Version]
- Boyle, N.K.; Pitts-Singer, T.L. Assessing blue orchard bee (Osmia lignaria) propagation and pollination services in the presence of honey bees (Apis mellifera) in Utah tart cherries. PeerJ 2019, 7, e7639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzschuh, A.; Dudenhöffer, J.H.; Tscharntke, T. Landscapes with wild bee habitats enhance pollination, fruit set and yield of sweet cherry. Biol. Conserv. 2012, 153, 101–107. [Google Scholar] [CrossRef]
- Bosch, J.; Osorio-Canadas, S.; Sgolastra, F.; Vicens, N. Use of a managed solitary bee to pollinate almonds: Population sustainability and increased fruit set. Insects 2021, 12, 56. [Google Scholar] [CrossRef]
- Peña, J.F.; Carabalí, A. Effect of honey bee (Apis mellifera L.) density on pollination and fruit set of avocado (Persea Americana Mill.) cv. Hass. J. Apic. Sci. 2018, 62, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Popak, A.E.; Markwith, S.H.; Strange, J. Economic valuation of bee pollination services for passion fruit (Malpighiales: Passifloraceae) cultivation on smallholding Farms in São Paulo, Brazil, using the avoided cost method. J. Econ. Entomol. 2019, 112, 2049–2054. [Google Scholar] [CrossRef]
- Malerbo-Souza, D.T.; Nogueira-Couto, R.H.; Couto, L.A. Honey bee attractants and pollination in sweet orange, Citrus sinensis (L.) Osbeck, var. Pera-Rio. J. Venom. Anim. Toxins Incl. Trop. Dis. 2004, 10, 144–153. [Google Scholar] [CrossRef]
- Deuri, A.; Rahman, A.; Gogoi, J.; Borah, P.; Bathari, M. Pollinator diversity and effect of Apis cerana F. pollination on yield of mango (Mangifera indica L.). J. Entomol. Zool. Stud. 2018, 6, 957–961. [Google Scholar]
- Klatt, B.K.; Holzschuh, A.; Westphal, C.; Clough, Y.; Smit, I.; Pawelzik, E.; Tscharntke, T. Bee pollination improves crop quality, shelf life and commercial value. Proc. Biol. Sci. 2014, 281, 20132440–20132447. [Google Scholar] [CrossRef]
- Herrmann, J.D.; Beye, H.; de la Broise, C.; Hartlep, H.; Diekötter, T. Positive effects of the pollinators Osmia cornuta (Megachilidae) and Lucilia sericata (Calliphoridae) on strawberry quality. Arthropod. Plant. Interact. 2019, 13, 71–77. [Google Scholar] [CrossRef]
- Howpage, D.; Spooner-Hart, R.N.; Vithanage, V. Influence of honey bee (Apis mellifera) on kiwifruit pollination and fruit quality under Australian conditions. New Zeal. J. Crop Hortic. Sci. 2001, 29, 51–59. [Google Scholar] [CrossRef]
- Greenleaf, S.S.; Kremen, C. Wild bee species increase tomato production and respond differently to surrounding land use in Northern California. Biol. Conserv. 2006, 133, 81–87. [Google Scholar] [CrossRef]
- Neto, S.; Lima, F.G.; Gonçalves, B.B.; Lima Bergamini, L.; Araújo, B.; Bergamini, R.; Antônio, M.; Elias, S.; Franceschinelli, E.V. Native bees pollinate tomato flowers and increase fruit production. J. Pollinat. Ecol. 2013, 11, 41–45. [Google Scholar]
- Munawar, M.S.; Sarwar, G.; Raja, S.; Waghchoure, E.S.; Iftikhar, F.; Mahmood, R. Pollination by honeybee (Apis mellifera) increases seed setting and yield in black seed (Nigella sativa). Int. J. Agric. Biol. 2009, 11, 611–615. [Google Scholar]
- Radford, B.J.; Nielsen, R.G.H.; Rhodes, J.W. Agents of pollination in sunflower crops on the central darling downs, queensland. Aust. J. Exp. Agric. 1979, 19, 565–569. [Google Scholar] [CrossRef]
- Pires, V.C.; Silveira, F.A.; Sujii, E.R.; Torezani, K.R.S.; Rodrigues, W.A.; Albuquerque, F.A.; Rodrigues, S.M.M.; Salomao, A.N.; Soares Pires, C.S. Importance of bee pollination for cotton production in conventional and organic farms in Brazil. J. Pollinat. Ecol. 2014, 13, 151–160. [Google Scholar] [CrossRef]
- Nicodemo, D.; Couto, R.H.N.; Malheiros, E.B.; de Jong, D. Honey bee as an effective pollinating agent of pumpkin. Sci. Agric. 2009, 66, 476–480. [Google Scholar] [CrossRef] [Green Version]
- Blettler, D.C.; Fagúndez, G.A.; Caviglia, O.P. Contribution of honeybees to soybean yield. Apidologie 2018, 49, 101–111. [Google Scholar] [CrossRef] [Green Version]
- De Milfont, M.O.; Rocha, E.E.M.; Lima, A.O.N.; Freitas, B.M. Higher soybean production using honeybee and wild pollinators, a sustainable alternative to pesticides and autopollination. Environ. Chem. Lett. 2013, 11, 335–341. [Google Scholar] [CrossRef]
- Krishnan, S.; Kushalappa, C.G.; Shaanker, R.U.; Ghazoul, J. Status of pollinators and their efficiency in coffee fruit set in a fragmented landscape mosaic in South India. Basic Appl. Ecol. 2012, 13, 277–285. [Google Scholar] [CrossRef]
- Asiwe, J.A.N. Insect mediated outcrossing and geneflow in cowpea (Vigna unguiculata (L.) Walp): Implication for seed production and provision of containment structures for genetically transformed cowpea. African J. Biotechnol. 2009, 8, 226–230. [Google Scholar] [CrossRef]
- Barrios, B.; Pena, S.R.; Salas, A.; Koptur, S. Butterflies visit more frequently, but bees are better pollinators: The importance of mouthpart dimensions in effective pollen removal and deposition. AoB Plants 2016, 8. [Google Scholar] [CrossRef] [Green Version]
- Mandal, E.; Amin, M.R.; Rahman, H.; Akanda, A.M. Abundance and foraging behavior of native insect pollinators and their effect on mustard (Brassica juncea L.). Bangladesh J. Zool 2018, 46, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Stanley, J.; Sah, K.; Subbanna, A.R.N.S. How efficient is the Asian honey bee, Apis cerana in pollinating mustard, Brassica campestris var. toria? Pollination behavior, pollinator efficiency, pollinator requirements and impact of pollination. J. Apic. Res. 2017, 56, 439–451. [Google Scholar] [CrossRef]
- Klein, A.M.; Steffan-Dewenter, I.; Tscharntke, T. Fruit set of highland coffee increases with the diversity of pollinating bees. Proc. R. Soc. B Biol. Sci. 2003, 270, 955–961. [Google Scholar] [CrossRef] [Green Version]
- Muto, N.A.; Leite, R.O. de S.; Pereira, D.S.; Rogez, H.L.G.; Venturieri, G.C. Impact of the introduction of stingless bee colonies (Scaptotrigona aff. postica) on the productivity of acai (Euterpe oleracea). Rev. Verde Agroecol. Desenvolv. Sustentável 2020, 15, 265–273. [Google Scholar] [CrossRef]
- Jauker, F.; Bondarenko, B.; Becker, H.C.; Steffan-Dewenter, I. Pollination efficiency of wild bees and hoverflies provided to oilseed rape. Agric. For. Entomol. 2012, 14, 81–87. [Google Scholar] [CrossRef]
- Perrot, T.; Gaba, S.; Roncoroni, M.; Gautier, J.L.; Bretagnolle, V. Bees increase oilseed rape yield under real field conditions. Agric. Ecosyst. Environ. 2018, 266, 39–48. [Google Scholar] [CrossRef]
- Bloch, D.; Werdenberg, N.; Erhardt, A. Pollination crisis in the butterfly-pollinated wild carnation Dianthus carthusianorum? New Phytol. 2006, 169, 699–706. [Google Scholar] [CrossRef]
- Pashte, V.V.; Kulkarni, S.R. Role of pollinators in qualitative fruit crop production: A review. Trends Biosci. 2015, 8, 3743–3749. [Google Scholar]
- Papiorek, S.; Junker, R.R.; Alves-dos-Santos, I.; Melo, G.A.R.; Amaral-Neto, L.P.; Sazima, M.; Wolowski, M.; Freitas, L.; Lunau, K. Bees, birds and yellow flowers: Pollinator-dependent convergent evolution of UV patterns. Plant Biol. 2016, 18, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.A.; Clayton, M.K.; Brunet, J. Floral traits influencing plant attractiveness to three bee species: Consequences for plant reproductive success. Am. J. Bot. 2017, 104, 772–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennessy, G.; Harris, C.; Eaton, C.; Wright, P.; Jackson, E.; Goulson, D.; Ratnieks, F.F.L.W. Gone with the wind: Effects of wind on honey bee visit rate and foraging behaviour. Anim. Behav. 2020, 161, 23–31. [Google Scholar] [CrossRef]
- Whitney, H.M.; Chittka, L.; Bruce, T.J.A.; Glover, B.J. Conical epidermal cells allow bees to grip flowers and increase foraging efficiency. Curr. Biol. 2009, 19, 948–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachersberger, M.; Cordeiro, G.D.; Schäffler, I.; Dötterl, S. Honeybee pollinators use visual and floral scent cues to find apple (Malus domestica) flowers. J. Agric. Food Chem. 2019, 67, 13221–13227. [Google Scholar] [CrossRef] [PubMed]
- Varassin, I.G.; Trigo, J.R.; Sazima, M. The role of nectar production, flower pigments and odour in the pollination of four species of Passiflora (Passifloraceae) in south-eastern Brazil. Bot. J. Linn. Soc. 2001, 136, 139–152. [Google Scholar] [CrossRef]
- Gresty, C.E.A.; Clare, E.; Devey, D.S.; Cowan, R.S.; Csiba, L.; Malakasi, P.; Lewis, O.T.; Willis, K.J. Flower preferences and pollen transport networks for cavity-nesting solitary bees: Implications for the design of agri-environment schemes. Ecol. Evol. 2018, 8, 7574–7587. [Google Scholar] [CrossRef]
- Prado, S.G.; Collazo, J.A.; Marand, M.H.; Irwin, R.E. The influence of floral resources and microclimate on pollinator visitation in an agro-ecosystem. Agric. Ecosyst. Environ. 2021, 307, 107196–107204. [Google Scholar] [CrossRef]
- Bradshaw, H.D.; Schemske, D.W. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature 2003, 426, 176–178. [Google Scholar] [CrossRef]
- Handelman, C.; Kohn, J.R. Hummingbird color preference within a natural hybrid population of Mimulus aurantiacus (Phrymaceae). Plant Species Biol. 2014, 29, 65–72. [Google Scholar] [CrossRef]
- Giurfa, M.; Núñez, J.; Chittka, L.; Menzel, R. Colour preferences of flower-naive honeybees. J. Comp. Physiol. A 1995, 177, 247–259. [Google Scholar] [CrossRef]
- Hill, P.S.M.; Wells, P.H.; Wells, H. Spontaneous flower constancy and learning in honey bees as a function of colour. Anim. Behav. 1997, 54, 615–627. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Liu, C.Q.; Sun, H.; Niu, Y. The ultraviolet colour component enhances the attractiveness of red flowers of a bee-pollinated plant. J. Plant Ecol. 2020, 13, 354–360. [Google Scholar] [CrossRef]
- Alcorn, K.; Whitney, H.; Glover, B. Flower movement increases pollinator preference for flowers with better grip. Funct. Ecol. 2012, 26, 941–947. [Google Scholar] [CrossRef]
- Whitney, H.M.; Poetes, R.; Steiner, U.; Chittka, L.; Glover, B.J. Determining the contribution of epidermal cell shape to petal wettability using isogenic antirrhinum lines. PLoS ONE 2011, 6, e17576. [Google Scholar] [CrossRef]
- Giuliani, C.; Giovanetti, M.; Lupi, D.; Mesiano, M.P.; Barilli, R.; Ascrizzi, R.; Flamini, G.; Fico, G. Tools to tie: Flower characteristics, voc emission profile, and glandular trichomes of two mexican salvia species to attract bees. Plants 2020, 9, 1645. [Google Scholar] [CrossRef]
- Solís-Montero, L.; Cáceres-García, S.; Alavez-Rosas, D.; García-Crisóstomo, J.F.; Vega-Polanco, M.; Grajales-Conesa, J.; Cruz-López, L. Pollinator preferences for floral volatiles emitted by dimorphic anthers of a buzz-pollinated herb. J. Chem. Ecol. 2018, 44, 1058–1067. [Google Scholar] [CrossRef]
- Makino, T.T.; Ohashi, K.; Sakai, S. How do floral display size and the density of surrounding flowers influence the likelihood of bumble bee revisitation to a plant? Funct. Ecol. 2007, 21, 87–95. [Google Scholar] [CrossRef]
- Shrestha, M.; Garcia, J.E.; Burd, M.; Dyer, A.G. Australian native flower colours: Does nectar reward drive bee pollinator flower preferences? PLoS ONE 2020, 15, e0226469. [Google Scholar] [CrossRef]
- Perera, R.A.S.N.; Karunaratne, W.A.I.P. Floral visits of the wild bee, lithurgus atratus, impact yield and seed germinability of okra, abelmoschus esculentus, in sri lanka. Pollinat. Ecol. 2019, 25, 1–6. [Google Scholar] [CrossRef]
- Mallinger, R.E.; Prasifka, J.R. Bee visitation rates to cultivated sunflowers increase with the amount and accessibility of nectar sugars. J. Appl. Entomol. 2017, 141, 561–573. [Google Scholar] [CrossRef]
- Rowe, L.; Gibson, D.; Bahlai, C.A.; Gibbs, J.; Landis, D.A.; Isaacs, R. Flower traits associated with the visitation patterns of bees. Oecologia 2020, 193, 511–522. [Google Scholar] [CrossRef]
- Robertson, A.W.; Mountjoy, C.; Faulkner, B.E.; Roberts, M.V.; Macnair, M.R. Bumble bee selection of Mimulus guttatus flowers: The effects of pollen quality and reward depletion. Ecology 1999, 80, 2594–2606. [Google Scholar] [CrossRef]
- Mallinger, R.E.; Franco, J.G.; Prischmann-Voldseth, D.A.; Prasifka, J.R. Annual cover crops for managed and wild bees: Optimal plant mixtures depend on pollinator enhancement goals. Agric. Ecosyst. Environ. 2019, 273, 107–116. [Google Scholar] [CrossRef]
- Urbanowicz, C.; Muñiz, P.A.; McArt, S.H. Honey bees and wild pollinators differ in their preference for and use of introduced floral resources. Ecol. Evol. 2020, 10, 6741–6751. [Google Scholar] [CrossRef]
- Ropars, L.; Dajoz, I.; Fontaine, C.; Muratet, A.; Geslin, B. Wild pollinator activity negatively related to honey bee colony densities in urban context. PLoS ONE 2019, 14, e0222316. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, D.J.; Vallejo-Marín, M. Floral vibrations by buzz-pollinating bees achieve higher frequency, velocity and acceleration than flight and defence vibrations. J. Exp. Biol. 2020, 223, jeb220541. [Google Scholar] [CrossRef]
- Pritchard, D.J.; Vallejo-marín, M. Quick guide Buzz pollination ll. Curr. Biol. 2020, 30, R858–R860. [Google Scholar] [CrossRef]
- De Luca, P.A.; Cox, D.A.; Vallejo-marín, M. Comparison of pollination and defensive buzzes in bumblebees indicates species-specific and context-dependent vibrations. Naturwissenschaften 2014, 101, 331–338. [Google Scholar] [CrossRef]
- Vallejo-Marín, M. Buzz pollination: Studying bee vibrations on flowers. New Phytol. 2019, 224, 1068–1074. [Google Scholar] [CrossRef] [Green Version]
- Russell, A.L.; Leonard, A.S.; Gillette, H.D.; Papaj, D.R. Concealed floral rewards and the role of experience in floral sonication by bees. Anim. Behav. 2016, 120, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Corbet, S.A.; Huang, S.-Q. Buzz pollination in eight bumblebee-pollinated Pedicularis species: Does it involve vibration-induced triboelectric charging of pollen grains? Ann. Bot. 2014, 114, 1665–1674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardinal, S.; Buchmann, S.L.; Russell, A.L. The evolution of floral sonication, a pollen foraging behavior used by bees (Anthophila). Evolution 2018, 72, 590–600. [Google Scholar] [CrossRef] [Green Version]
- Wcislo, W.T.; Tierney, S.M. Behavioural environments and niche construction: The evolution of dim-light foraging in bees. Biol. Rev. 2009, 84, 19–37. [Google Scholar] [CrossRef]
- Ma, W.; Li, X.; Shen, J.; Du, Y.; Xu, K.; Jiang, Y. Transcriptomic analysis reveals Apis mellifera adaptations to high temperature and high humidity. Ecotoxicol. Environ. Saf. 2019, 184, 109599. [Google Scholar] [CrossRef]
- Dolezal, A.G.; Toth, A.L. Feedbacks between nutrition and disease in honey bee health. Curr. Opin. Insect Sci. 2018, 26, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.Y.; Giray, T. Factors affecting pollinators and pollination. Psyche 2012, 2012. [Google Scholar] [CrossRef]
- Christopher Brown, J.; Albrecht, C. The effect of tropical deforestation on stingless bees of the genus Melipona (Insecta: Hymenoptera: Apidae: Meliponini) in central Rondonia, Brazil. J. Biogeogr. 2001, 28, 623–634. [Google Scholar] [CrossRef]
- Yang, D.; Xu, X.; Zhao, H.; Yang, S.; Wang, X.; Zhao, D.; Diao, Q.; Hou, C. Diverse factors affecting efficiency of RNAi in honey bee viruses. Front. Genet. 2018, 9, 384–392. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, S.T.; Swale, D.R.; Anderson, T.D. ATP-sensitive inwardly rectifying potassium channel regulation of viral infections in honey bees. Sci. Rep. 2017, 7, 8668–8676. [Google Scholar] [CrossRef] [Green Version]
- van Engelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony collapse disorder: A descriptive study. PLoS ONE 2009, 4, e6481. [Google Scholar] [CrossRef]
- Van Dooremalen, C.; van Langevelde, F. Can colony size of honeybees (Apis mellifera) be used as predictor for colony losses due to varroa destructor during winter? Agriculture1 2021, 11, 529. [Google Scholar] [CrossRef]
- Snow, J.W.; Ceylan Koydemir, H.; Karinca, D.K.; Liang, K.; Tseng, D.; Ozcan, A. Rapid imaging, detection, and quantification of Nosema ceranae spores in honey bees using mobile phone-based fluorescence microscopy. Lab Chip 2019, 19, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.J.; Palmer-Young, E.C.; Irwin, R.E.; Adler, L.S. Food limitation affects parasite load and survival of Bombus impatiens (Hymenoptera: Apidae) infected with Crithidia (Trypanosomatida: Trypanosomatidae). Environ. Entomol. 2016, 45, 1212–1219. [Google Scholar] [CrossRef]
- Han, W.; Yang, Y.; Gao, J.; Zhao, D.; Ren, C.; Wang, S.; Zhao, S.; Zhong, Y. Chronic toxicity and biochemical response of Apis cerana cerana (Hymenoptera: Apidae) exposed to acetamiprid and propiconazole alone or combined. Ecotoxicology 2019, 28, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Christen, V.; Kunz, P.Y.; Fent, K. Endocrine disruption and chronic effects of plant protection products in bees: Can we better protect our pollinators? Environ. Pollut. 2018, 243, 1588–1601. [Google Scholar] [CrossRef] [PubMed]
- Arce, A.N.; Ramos Rodrigues, A.; Yu, J.; Colgan, T.J.; Wurm, Y.; Gill, R.J. Foraging bumblebees acquire a preference for neonicotinoid-treated food with prolonged exposure. Proceedings. Biol. Sci. 2018, 285, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, B.A.; Isaac, N.J.B.; Bullock, J.M.; Roy, D.B.; Garthwaite, D.G.; Crowe, A.; Pywell, R.F. Impacts of neonicotinoid use on long-term population changes in wild bees in England. Nat. Commun. 2016, 7, 12459. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Ma, D.; Zou, N.; Yu, X.; Zhang, Z.; Liu, F.; Mu, W. Concentrations of Imidacloprid and Thiamethoxam in Pollen, Nectar and Leaves from seed-Dressed Cotton Crops and their Potential Risk to Honeybees (Apis mellifera L.); Elsevier Ltd.: Amsterdam, The Netherlands, 2018; Volume 201, ISBN 8605388242. [Google Scholar]
- Tosi, S.; Nieh, J.C.; Sgolastra, F.; Cabbri, R.; Medrzycki, P. Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honey bees. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171711–20171719. [Google Scholar] [CrossRef] [Green Version]
- Tomé, H.V.V.; Ramos, G.S.; Araújo, M.F.; Santana, W.C.; Santos, G.R.; Guedes, R.N.C.; Maciel, C.D.; Newland, P.L.; Oliveira, E.E. Agrochemical synergism imposes higher risk to neotropical bees than to honeybees. R. Soc. Open Sci. 2017, 4, 160866. [Google Scholar] [CrossRef] [Green Version]
- Vanegas, M. The silent beehive: How the decline of honey bee populations shifted the environmental protection agency’s pesticide policy towards pollinators. Ecol. Law Q. 2017, 44, 311–342. [Google Scholar]
- Rortais, A.; Arnold, G.; Dorne, J.L.; More, S.J.; Sperandio, G.; Streissl, F.; Szentes, C.; Verdonck, F. Risk assessment of pesticides and other stressors in bees: Principles, data gaps and perspectives from the European Food Safety Authority. Sci. Total Environ. 2017, 587–588, 524–537. [Google Scholar] [CrossRef]
- Tschoeke, P.H.; Oliveira, E.E.; Dalcin, M.S.; Silveira-Tschoeke, M.C.A.C.; Sarmento, R.A.; Santos, G.R. Botanical and synthetic pesticides alter the flower visitation rates of pollinator bees in Neotropical melon fields. Environ. Pollut. 2019, 251, 591–599. [Google Scholar] [CrossRef]
- Liporoni, R.; Cordeiro, G.D.; Prado, P.I.; Schlindwein, C.; Warrant, E.J.; Alves-dos-Santos, I. Light intensity regulates flower visitation in Neotropical nocturnal bees. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Kenefic, N.E.; González, P.L.; Theodorou, P.; Cardona, E. Disentangling the effects of local resources, landscape heterogeneity and climatic seasonality on bee diversity and plant—Pollinator networks in tropical highlands. Oecologia 2020, 194, 333–344. [Google Scholar] [CrossRef]
- Grassl, J.; Holt, S.; Cremen, N.; Peso, M.; Hahne, D.; Baer, B. Synergistic effects of pathogen and pesticide exposure on honey bee (Apis mellifera) survival and immunity. J. Invertebr. Pathol. 2018, 159, 78–86. [Google Scholar] [CrossRef]
- Dalsgaard, B. Land-use and climate impacts on plant-pollinator interactions and pollination services. Diversity 2020, 12, 168. [Google Scholar] [CrossRef]
- Jacques, A.; Laurent, M.; Consortium, E.; Ribière-Chabert, M.; Saussac, M.; Bougeard, S.; Budge, G.E.; Hendrikx, P.; Chauzat, M.-P. A pan-European epidemiological study reveals honey bee colony survival depends on beekeeper education and disease control. PLoS ONE 2017, 12, e0172591. [Google Scholar] [CrossRef] [Green Version]
- Cook, D.F.; Voss, S.C.; Finch, J.T.D.; Rader, R.C.; Cook, J.M.; Spurr, C.J. The role of flies as pollinators of horticultural crops: An Australian case study with worldwide relevance. Insects 2020, 11, 341. [Google Scholar] [CrossRef]
- Larson, B.M.H.; Kevan, P.G.; Inouye, D.W. Flies and flowers: Taxonomic diversity of anthophiles and pollinators. Can. Entomol. 2001, 133, 439–465. [Google Scholar] [CrossRef] [Green Version]
- Hodgkiss, D.; Brown, M.J.F.; Fountain, M.T. Syrphine hoverflies are effective pollinators of commercial strawberry. J. Pollinat. Ecol. 2018, 22, 55–66. [Google Scholar] [CrossRef]
- Jauker, F.; Wolters, V. Hover flies are efficient pollinators of oilseed rape. Oecologia 2008, 156, 819–823. [Google Scholar] [CrossRef]
- Howlett, B.G.; Gee, M. The potential management of the drone fly (Eristalis tenax) as a crop pollinator in New Zealand. N. Z. Plant Prot. 2019, 72, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Schittenhelm, S.; Gladis, T.; Rao, V.R. Efficiency of various insects in germplasm regeneration of carrot, onion and turnip rape accessions. Plant Breed. 1997, 116, 369–375. [Google Scholar] [CrossRef]
- Jarlan, A.; De Oliveira, D.; Gingras, J. Pollination of sweet pepper (Capsicum annuum L.) in green-house by the syrphid fly Eristalis tenax (L.). Acta Hortic. 1997, 437, 335–339. [Google Scholar] [CrossRef]
- Barbattini, R.; Greatti, M.; Zandigiacomo, P.; Costa, G.; Testolin, R.; Vizzotto, G. Insect pollinators of kiwifruit and their role in crop pollination. In Proceedings of the Atti XVII Congresso Nazionale Italiano di Entomologia, Udine, Italy, 13–18 June 1994; pp. 13–18. [Google Scholar]
- Hahn, M.; Brühl, C.A. The secret pollinators: An overview of moth pollination with a focus on Europe and North America. Arthropod. Plant. Interact. 2016, 10, 21–28. [Google Scholar] [CrossRef]
- Santos, R.d.S.; Milfont, M.d.O.; Silva, M.M.; Carneiro, L.T.; Castro, C.C. Butterflies provide pollination services to macadamia in northeastern Brazil. Sci. Hortic. 2020, 259, 108818–108825. [Google Scholar] [CrossRef]
- Martins, D.J. Butterfly pollination of the dryland wildflower gloriosa minor. J. East African Nat. Hist. 2014, 103, 25–30. [Google Scholar] [CrossRef]
- Cruden, R.W.; Hermann-parker, S.M. Butterfly pollination of Caesalpinia pulcherrima, with observations on a psychophilous syndrome. Br. Ecol. Soc. 1979, 67, 155–168. [Google Scholar] [CrossRef]
- Pinto, C.E.; Oliveira, R.; Schlindwein, C. Do consecutive flower visits within a crown diminish fruit set in mass-flowering Hancornia speciosa (Apocynaceae)? Plant Biol. 2008, 10, 408–412. [Google Scholar] [CrossRef]
- Barrios, B.; Koptur, S. Floral biology and breeding system of Angadenia berteroi (Apocynaceae): Why do flowers of the pineland golden trumpet produce few fruits? Int. J. Plant Sci. 2011, 172, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Gann, G.D.; Woodmansee, S.W.; Bradley, K.A. Conserving rare plants in locally-protected urban forest fragments: A case study from Miami-Dade County, Florida. Urban For. Urban Greening. 2020, 20, 1–11. [Google Scholar]
- Macgregor, C.J.; Pocock, M.J.O.; Fox, R.; Evans, D.M. Pollination by nocturnal Lepidoptera, and the effects of light pollution: A review. Ecol. Entomol. 2015, 40, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Ricketts, T.H.; Regetz, J.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Bogdanski, A.; Gemmill-Herren, B.; Greenleaf, S.S.; Klein, A.M.; Mayfield, M.M.; et al. Landscape effects on crop pollination services: Are there general patterns? Ecol. Lett. 2008, 11, 499–515. [Google Scholar] [CrossRef]
- Power, A.G. Ecosystem services and agriculture: Tradeoffs and synergies. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2959–2971. [Google Scholar] [CrossRef]
- Alarcón, R.; Davidowitz, G.; Bronstein, J.L. Nectar usage in a southern Arizona hawkmoth community. Ecol. Entomol. 2008, 33, 503–509. [Google Scholar] [CrossRef]
- Devoto, M.; Bailey, S.; Memmott, J. The “night shift”: Nocturnal pollen-transport networks in a boreal pine forest. Ecol. Entomol. 2011, 36, 25–35. [Google Scholar] [CrossRef]
- Sime, K.R.; Baldwin, I.T. Predominantly self-fertilizing native tobacco. BMC Ecol. 2003, 3, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, J.E.; Irvine, A.K. Floral biology of Myristica insipida (Myristicaceae), a distinctive beetle pollination syndrome. Am. J. Bot. 1989, 76, 86–94. [Google Scholar] [CrossRef]
- Bernhardt, P. Convergent evolution and adaptive radiation of beetle-pollinated angiosperms. Pl Syst. Evol. 2000, 222, 293–320. [Google Scholar] [CrossRef]
- Dieringer, G.; Cabrera, R.L.; Lara, M.; Loya, L.; Reyes, P.; Journal, I. Beetle pollination and floral thermogenicity in Magnolia tamaulipana (Magnoliaceae). Int. J. Plant Sci. 1999, 160, 64–71. [Google Scholar] [CrossRef]
- Mayer, C.; Soka, G.; Picker, M. The importance of monkey beetle (Scarabaeidae: Hopliini) pollination for Aizoaceae and Asteraceae in grazed and ungrazed areas at Paulshoek, Succulent Karoo, South Africa. J. Insect Conserv. 2006, 10, 323–333. [Google Scholar] [CrossRef]
- Devy, M.S.; Davidar, P. Pollination systems of trees in Karachi, a mid-elevation wet evergreen forest in Western Ghats, India. Am. J. Bot. 2003, 90, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Momose, K.; Yumoto, T.; Nagamitsu, T.; Kato, M.; Nagamasu, H.; Sakai, S.; Harrison, R.D.; Itioka, T.; Hamid, A.A.; Inoue, T. Pollination biology in a lowland dipterocarp forest in Sarawak, Malaysia. I. Characteristics of the plant-pollinator community in a lowland dipterocarp forest. Am. J. Bot. 1998, 85, 1477–1501. [Google Scholar] [CrossRef]
- Colville, J.; Picker, M.D.; Cowling, R.M. Species turnover of monkey beetles (Scarabaeidae: Hopliini) along environmental and disturbance gradients in the Namaqualand region of the succulent Karoo, South Africa. Biodivers. Conserv. 2002, 11, 243–264. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Nänni, I.; Donaldson, J.S.; Manning, J.C. The role of beetle marks and flower colour on visitation by monkey beetles (Hopliini) in the greater c\ape Floral Region, South Africa. Ann. Bot. 2007, 100, 1483–1489. [Google Scholar] [CrossRef] [Green Version]
- Sayers, T.D.J.; Steinbauer, M.J.; Miller, R.E. Visitor or vector? The extent of rove beetle (Coleoptera: Staphylinidae) pollination and floral interactions. Arthropod. Plant. Interact. 2019, 13, 685–701. [Google Scholar] [CrossRef]
- Suetsugu, K. Social wasps, crickets and cockroaches contribute to pollination of the holoparasitic plant Mitrastemon yamamotoi (Mitrastemonaceae) in southern Japan. Plant Biol. 2019, 21, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Ananthakrishnan, T.N. Thrips and pollination biology. Curr. Sci. 1982, 51, 168–172. [Google Scholar]
- Infante, F.; Ortíz, J.A.; Solis-Montero, L.; Mound, L.A.; Vega, F.E. Thrips (Thysanoptera) of coffee flowers. Ann. Entomol. Soc. Am. 2017, 110, 329–336. [Google Scholar] [CrossRef]
- Resende, J.J.; de M. Santos, G.M.; Bichara Filho, C.C.; Gimenes, M. Atividade diária de busca de recursos pela vespa social Polybia occidentalis occidentalis (Olivier, 1791) (Hymenoptera, Vespidae). Rev. Bras. Zoociências 2001, 3, 105–115. [Google Scholar]
- Beggs, J. The ecological consequences of social wasps (Vespula spp.) invading an ecosystem that has an abundant carbohydrate resource. Biol. Conserv. 2001, 99, 17–28. [Google Scholar] [CrossRef]
- Schremmer, F. Wespen und Hornissen; Ziemsen: Wittenberg Lutherstadt, Germany, 1962. [Google Scholar]
- Brodmann, J.; Twele, R.; Francke, W.; Hölzler, G.; Zhang, Q.H.; Ayasse, M. Orchids mimic green-leaf volatiles to attract prey-hunting wasps for pollination. Curr. Biol. 2008, 18, 740–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakubska-Busse, A.; Przado, D.; Steininger, M.; Aniolł-Kwiatkowska, J.; Kadej, M. Why do pollinators become “sluggish”? Nectar chemical constituents from Epipactis helleborine (L.) crantz (Orchidaceae). Appl. Ecol. Environ. Res. 2005, 3, 29–38. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; AlAjmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Overview of Bee Pollination and Its Economic Value for Crop Production. Insects 2021, 12, 688. https://doi.org/10.3390/insects12080688
Khalifa SAM, Elshafiey EH, Shetaia AA, El-Wahed AAA, Algethami AF, Musharraf SG, AlAjmi MF, Zhao C, Masry SHD, Abdel-Daim MM, et al. Overview of Bee Pollination and Its Economic Value for Crop Production. Insects. 2021; 12(8):688. https://doi.org/10.3390/insects12080688
Chicago/Turabian StyleKhalifa, Shaden A. M., Esraa H. Elshafiey, Aya A. Shetaia, Aida A. Abd El-Wahed, Ahmed F. Algethami, Syed G. Musharraf, Mohamed F. AlAjmi, Chao Zhao, Saad H. D. Masry, Mohamed M. Abdel-Daim, and et al. 2021. "Overview of Bee Pollination and Its Economic Value for Crop Production" Insects 12, no. 8: 688. https://doi.org/10.3390/insects12080688