Egg Sterilisation of Irradiated Nezara viridula (Hemiptera: Pentatomidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
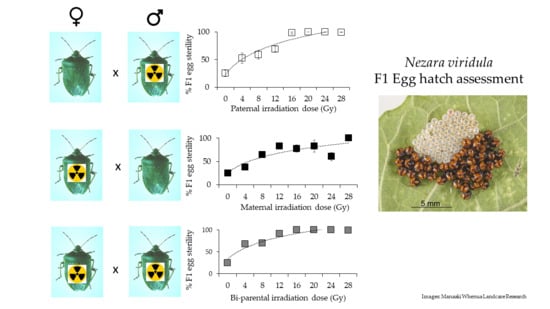
3. Results
4. Discussion
4.1. Development of SIT for N. viridula and Pentatomid Pests
4.2. Application of SIT for N. viridula and Other Pentatomid Pests
4.3. Data Gap—F2 Mortality
4.4. Future Needs
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Todd, J.W. Ecology and behavior of Nezara Viridula. Annu. Rev. Entomol. 1989, 34, 273–292. [Google Scholar] [CrossRef]
- Rea, J.H.; Cameron, P.J.; Wratten, S.D.; Davis, S.I.; Sedcole, J.R.; Chapman, R.B. Evaluation of insecticides for the control of the green vegetable bug, Nezara viridula (L.) (Hemiptera: Pentatomidae), on sweet corn, Zea mays (L.), in New Zealand. Int. J. Pest. Manag. 2003, 49, 105–108. [Google Scholar] [CrossRef]
- Panizzi, A.R.; Lucini, T. What happened to Nezara viridula (L.) in the Americas? Possible reasons to explain populations decline. Neotrop. Entomol. 2016, 45, 619–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara, J.R.; Pickett, C.; Hannon, E.; Gonzalez, L.; Figuera, S.; Romo, M.; Cabanas, C.; Bazurto, V.; Vincent, S.; Briseno, K.; et al. Trouble comes in pairs: Invasive stink bugs in California. CAPCA Adviser 2018, 21, 62–72. [Google Scholar]
- Panizzi, A.R. Wild hosts of pentatomids: Ecological significance and role in their pest status on crops. Annu. Rev. Entomol. 1997, 42, 99–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willrich, M.M.; Temple, J.; Gable, R.H.; Leonard, B.R. Evaluation of insecticides for control of nymph and adult southern green stink bugs, 2002. Arthropod Manag. Tests 2003, 28, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Tillman, P.G. Susceptibility of pest Nezara viridula (Heteroptera: Pentatomidae) and parasitoid Trichopoda pennipes (Diptera: Tachinidae) to selected insecticides. J. Econ. Entomol. 2006, 99, 10. [Google Scholar] [CrossRef]
- Knight, K.M.M.; Gurr, G.M. Review of Nezara viridula (L.) management strategies and potential for IPM in field crops with emphasis on Australia. Crop Prot. 2007, 26, 1–10. [Google Scholar] [CrossRef]
- Čokl, A.A.; Millar, J.G. Manipulation of insect signaling for monitoring and control of pest insects. In Biorational Control of Arthropod Pests; Ishaaya, I., Horowitz, A.R., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 279–316. ISBN 978-90-481-2315-5. [Google Scholar]
- Mazzoni, V.; Polajnar, J.; Baldini, M.; Rossi Stacconi, M.V.; Anfora, G.; Guidetti, R.; Maistrello, L. Use of substrate-borne vibrational signals to attract the brown marmorated stink bug, Halyomorpha halys. J. Pest Sci. 2017, 90, 1219–1229. [Google Scholar] [CrossRef]
- Welsh, T.J.; Stringer, L.D.; Caldwell, R.; Carpenter, J.E.; Suckling, D.M. Irradiation biology of male brown marmorated stink bugs: Is there scope for the sterile insect technique? Int. J. Radiat. Biol. 2017, 93, 1357–1363. [Google Scholar] [CrossRef]
- Nagel, P.; Peveling, R. Environment and the sterile insect technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 499–519. [Google Scholar]
- Klassen, W.; Curtis, C.F. History of the sterile insect technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 3–38. [Google Scholar]
- Carpenter, J.E.; Bloem, S.; Hofmeyr, J.H. Inherited sterility in insects. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 115–146. [Google Scholar]
- Mau, R.; Mitchell, W.C.; Anwar, M. Preliminary studies on the effects of gamma irradiation of eggs and adults of the southern green stink bug, Nezara viridula (L.). Proc. Hawaii. Entomol. Soc. 1967, 19, 415–417. [Google Scholar]
- Dyby, S.D.; Sailer, R.I. Impact of low-level radiation on fertility and fecundity of Nezara Viridula (Hemiptera: Pentatomidae). J. Econ. Entomol. 1999, 92, 945–953. [Google Scholar] [CrossRef]
- Žunič, A.; Čokl, A.; Serša, G. Effects of 5-Gy irradiation on fertility and mating behaviour of Nezara viridula (Heteroptera: Pentatomidae). Radiol. Oncol. 2002, 36, 231–237. [Google Scholar]
- Hallman, G.J.; Chapa, D.L. Phytosanitary Irradiation of Diaphorina citri (Hemiptera: Liviidae). Fla. Entomol. 2016, 99, 150–152. [Google Scholar]
- International Atomic Energy Agency International Database on Insect Disinfestation and Sterilization. Available online: https://nucleus.iaea.org/sites/naipc/ididas/Pages/Browse-IDIDAS.aspx (accessed on 30 March 2020).
- Tadic, M. Sterilisation of overwintering adults of the sun pest. Zast. Bilga 1972, 23, 65–71. [Google Scholar]
- Suckling, D.M.; Levy, M.C.; Roselli, G.; Mazzoni, V.; Ioriatti, C.; Deromedi, M.; Cristofaro, M.; Anfora, G. Live traps for adult brown marmorated stink bugs. Insects 2019, 10, 376. [Google Scholar] [CrossRef] [Green Version]
- Suckling, D.M.; Cristofaro, M.; Roselli, G.; Levy, M.C.; Cemmi, A.; Mazzoni, V.; Stringer, L.D.; Zeni, V.; Ioriatti, C.; Anfora, G. The competitive mating of irradiated brown marmorated stink bugs, Halyomorpha halys, for the Sterile Insect Technique. Insects 2019, 10, 411. [Google Scholar] [CrossRef] [Green Version]
- Horrocks, K.J.; Avila, G.A.; Holwell, G.I.; Suckling, D.M. Integrating sterile insect technique with the release of sterile classical biocontrol agents for eradication: Is the kamikaze wasp technique feasible? BioControl 2020, 65, 257–271. [Google Scholar] [CrossRef]
- Parker, A.G. Mass-rearing for sterile insect release. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 209–232. [Google Scholar]
- Parker, A.; Mehta, K. Sterile insect technique: A model for dose optimization for improved sterile insect quality. Fla. Entomol. 2007, 90, 88–95. [Google Scholar] [CrossRef]
- Bakri, A.; Mehta, K.; Lance, D.R. Sterilizing insects with ionizing radiation. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 233–268. [Google Scholar]
- Williamson, D.L.; Mitchell, S.; Seo, S.T. Gamma irradiation of the Mediterranean fruit fly (Diptera: Tephritidae): Effects of puparial age under induced hypoxia on female sterility. Ann. Entomol. Soc. Am. 1985, 78, 101–106. [Google Scholar] [CrossRef]
- Srivastava, K.P.; Deshpande, D.J. Effect of x-irradiation on fecundity, fertility, and longevity of the red cotton bug Dysdercus koenigii Fabr. J. Exp. Biol. 1983, 21, 604–606. [Google Scholar]
- Srivastava, K.P.; Deshpande, D.J.; Katiyar, R.L. X-irradiation-induced histochemical changes in the ovaries of Dysdercus koenigii Fabr. Entomon 1985, 10, 29–33. [Google Scholar]
- Calvitti, M.; Govoni, C.; Butturazi, M.; Cirio, U. Induced sterility in greenhouse whitefly (Homoptera: Aleyrodidae) treated with gamma radiation. J. Econ. Entomol. 1997, 90, 1022–1027. [Google Scholar]
- Lance, D.R.; McInnis, D.O. Biological basis of the sterile insect technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 69–94. [Google Scholar]
- Oliva, C.F.; Jacquet, M.; Gilles, J.; Lemperiere, G.; Maquart, P.-O.; Quilici, S.; Schooneman, F.; Vreysen, M.J.B.; Boyer, S. The sterile insect technique for controlling populations of Aedes albopictus (Diptera: Culicidae) on Reunion Island: Mating vigour of sterilized males. PLoS ONE 2012, 7, e49414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanouette, G.; Brodeur, J.; Fournier, F.; Martel, V.; Vreysen, M.; Cáceres, C.; Firlej, A. The sterile insect technique for the management of the spotted wing drosophila, Drosophila suzukii: Establishing the optimum irradiation dose. PLoS ONE 2017, 12, e0180821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloem, S.; Bloem, K.A.; Carpenter, J.E.; Calkins, C.O. Inherited sterility in codling moth (Lepidoptera: Tortricidae): Effect of substerilizing doses of radiation on insect fecundity, fertility, and control. Ann. Entomol. Soc. Am. 1999, 92, 222–229. [Google Scholar] [CrossRef]
- Ayvaz, A.; Albayrak, S.; Tunçbilek, A.Ş. Inherited sterility in Mediterranean flour moth Ephestia kuehniella Zeller (Lepidoptera: Pyralidae): Effect of gamma radiation on insect fecundity, fertility and developmental period. J. Stored Prod. Res. 2007, 43, 234–239. [Google Scholar] [CrossRef]
- Blomefield, T.L.; Bloem, S.; Carpenter, J.E. Effect of radiation on fecundity and fertility of codling moth Cydia pomonella (Linnaeus) (Lepidoptera: Tortricidae) from South Africa. J. Appl. Entomol. 2010, 134, 216–220. [Google Scholar] [CrossRef]
- Jang, E.B.; McInnis, D.O.; Kurashima, R.; Woods, B.; Suckling, D.M. Irradiation of adult Epiphyas postvittana (Lepidoptera: Tortricidae): Egg sterility in parental and F1 generations. J. Econ. Entomol. 2012, 105, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, W.C.; Mau, R.F.L. Sexual activity and longevity of the southern green stink bug, Nezara viridula. Ann. Entomol. Soc. Am. 1969, 62, 1246–1247. [Google Scholar] [CrossRef]
- Harris, V.E.; Todd, J.W.; Mullinix, G. Color change as an indicator of adult diapause in the southern green stink bug, Nezara viridula. J. Agric. Entomol. 1984, 1, 82–91. [Google Scholar]
- Vivan, L.M.; Panizzi, A.R. Nymphal and adult performance of genetically determined types of Nezara viridula (L.) (Heteroptera: Pentatomidae), under different temperature and photoperiodic conditions. Neotrop. Entomol. 2005, 34, 911–915. [Google Scholar] [CrossRef] [Green Version]
- LaChance, L.E.; Degrugillier, M.; Leverich, A.P. Cytogenetics of inherited partial sterility in three generations of the large milkweed bug as related to holokinetic chromosomes. Chromosoma 1970, 29, 20–41. [Google Scholar] [CrossRef] [PubMed]
- Traut, W.; Sahara, K.; Marec, F. Sex chromosomes and sex determination in Lepidoptera. Sex. Dev. 2007, 1, 332–346. [Google Scholar] [CrossRef]
- Carpenter, J.E.; Marti, O.G.; Wee, S.L.; Suckling, D.M. Cytological attributes of sperm bundles unique to F1 progeny of irradiated male Lepidoptera: Relevance to sterile insect technique programs. Fla. Entomol. 2009, 92, 80–86. [Google Scholar] [CrossRef]
- Stringer, L.D.; Harland, D.; Grant, J.E.; Laban, J.; Suckling, D.M. Effect of 40Gy irradiation on the ultrastructure, biochemistry, morphology, and cytology during spermatogenesis in the southern green stink bug Nezara viridula (Hemiptera: Pentatomidae). BioRxiv 2017, 17, 1991. [Google Scholar]
- Saeed, R.; Abbas, N.; Razaq, M.; Mahmood, Z.; Naveed, M.; Rehman, H.M.U. Field evolved resistance to pyrethroids, neonicotinoids and biopesticides in Dysdercus koenigii (Hemiptera: Pyrrhocoridae) from Punjab, Pakistan. Chemosphere 2018, 213, 149–155. [Google Scholar] [CrossRef]
- Tamhankar, A.J.; Harwalker, M.R. Mating behaviour and mating competitiveness of radiation sterilized males of Dysdercus koenigii Fabricus. J. Nucl. Agric. Biol. 1995, 24, 180–184. [Google Scholar]
- Enkerlin, W.R. Impact of fruit fly control programmes using the sterile insect technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 651–676. [Google Scholar]
- Hight, S.D.; Carpenter, J.E.; Bloem, S.; Bloem, K.A. Developing a sterile insect release program for Cactoblastis cactorum (Berg) (Lepidoptera: Pyralidae): Effective overflooding ratios and release-recapture field studies. Environ. Entomol. 2005, 34, 850–856. [Google Scholar] [CrossRef] [Green Version]
- Hendrichs, J.; Franz, G.; Rendon, P. Increased effectiveness and applicability of the sterile insect technique through male-only releases for control of Mediterranean fruit flies during fruiting seasons. J. Appl. Entomol. 1995, 119, 371–377. [Google Scholar] [CrossRef]
- Bloem, K.A.; Bloem, S.; Carpenter, J.E. Impact of moth suppression/eradication programmes using the sterile insect technique or inherited sterility. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 677–700. [Google Scholar]
- Fortes, P.; Cônsoli, F.L. Are there costs in the repeated mating activities of female Southern stink bugs Nezara viridula? Physiol. Entomol. 2011, 36, 215–219. [Google Scholar] [CrossRef]
- Knipling, E.F. The Basic Principles of Insect Population Suppression and Management; Agriculture Handbook; United States Department of Agriculture: Washington, DC, USA, 1979.
- Kawada, H.; Kitamura, C. The reproductive behaviour of the brown marmorated stink bug, Halyomorpha mista Uhler (Heteroptera: Pentatomidae) I. Observation of mating behaviour and multiple copulation. Appl. Entomol. Zool. 1983, 18, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Haye, T.; Gariepy, T.; Hoelmer, K.; Rossi, J.-P.; Streito, J.-C.; Tassus, X.; Desneux, N. Range expansion of the invasive brown marmorated stinkbug, Halyomorpha halys: An increasing threat to field, fruit and vegetable crops worldwide. J. Pest Sci. 2015, 88, 665–673. [Google Scholar] [CrossRef]
- Kiritani, K.; Hokyo, N.; Kimura, K.; Nakasuji, F. Imaginal dispersal of the southern green stink bug, Nezara viridula L., in relation to feeding and oviposition. Jpn. J. Appl. Entomol. Z. 1965, 9, 291–297. [Google Scholar] [CrossRef]
- Panizzi, A.R.; Vivan, L.M.; Corrêa-Ferreira, B.S.; Foerster, L.A. Performance of southern green stink bug (Heteroptera: Pentatomidae) nymphs and adults on a novel food plant (Japanese privet) and other hosts. Ann. Entomol. Soc. Am. 1996, 89, 822–827. [Google Scholar] [CrossRef]
- Panizzi, A.R.; Parra, J.R.P.; Santos, C.H.; Carvalho, D.R. Rearing the southern green stink bug using an artificial dry diet and an artificial plant. Pesq. Agropec. Bras. 2000, 35, 1709–1715. [Google Scholar] [CrossRef] [Green Version]
- Medal, J.; Smith, T.; Fox, A.; Cruz, A.S.; Poplin, A.; Hodges, A. Rearing the brown marmorated stink bug Halyomorpha halys (Heteroptera: Pentatomidae). Fla. Entomol. 2012, 95, 800–802. [Google Scholar] [CrossRef]
- Jones, W.A.; Sullivan, M.J. Overwintering habitats, spring emergence patterns, and winter mortality of some South Carolina Hemiptera 1. Environ. Entomol. 1981, 10, 409–414. [Google Scholar] [CrossRef]
- Leskey, T.C.; Hamilton, G.C.; Nielsen, A.L.; Polk, D.F.; Rodriguez-Saona, C.; Bergh, J.C.; Herbert, D.A.; Kuhar, T.P.; Pfeiffer, D.; Dively, G.P.; et al. Pest status of the brown marmorated stink bug, Halyomorpha halys in the USA. Outlooks Pest Manag. 2012, 23, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Musolin, D.L. Surviving winter: Diapause syndrome in the southern green stink bug Nezara viridula in the laboratory, in the field, and under climate change conditions. Physiol. Entomol. 2012, 37, 309–322. [Google Scholar] [CrossRef]
- Bloem, S.; Bloem, K.A.; Fielding, L.S. Mass-rearing and storing codling moth larvae in diapause: A novel approach to increase production for sterile insect release. J. Entomol. Soc. B. C. 1997, 94, 75–81. [Google Scholar]
- Calkins, C.O.; Parker, A.G. Sterile insect quality. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 269–296. [Google Scholar]
- Simmons, G.S.; Suckling, D.M.; Carpenter, J.E.; Addison, M.F.; Dyck, V.A.; Vreysen, M.J.B. Improved quality management to enhance the efficacy of the sterile insect technique for lepidopteran pests. J. Appl. Entomol. 2010, 134, 261–273. [Google Scholar] [CrossRef]
- Fortes, P.; Magro, S.R.; Panizzi, A.R.; Parra, J.R. Development of a dry artificial diet for Nezara viridula (L.) and Euschistus heros (Fabricius) (Heteroptera: Pentatomidae). Neotrop. Entomol. 2006, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastrangelo, T.; Kovaleski, A.; Botteon, V.; Scopel, W.; Costa, M. de L.Z. Optimization of the sterilizing doses and overflooding ratios for the South American fruit fly. PLoS ONE 2018, 13, e0201026. [Google Scholar] [CrossRef] [PubMed]
- Kean, J.M.; Wee, S.L.; Stephens, A.E.A.; Suckling, D.M. Modelling the effects of inherited sterility for the application of the sterile insect technique. Agric. Forest Entomol. 2008, 10, 101–110. [Google Scholar] [CrossRef]
- Suckling, D.M.; Tobin, P.C.; McCullough, D.G.; Herms, D.A. Combining tactics to exploit Allee effects for eradication of alien insect populations. J. Econ. Entom. 2012, 105, 1–13. [Google Scholar] [CrossRef] [Green Version]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horrocks, K.J.; Welsh, T.; Carpenter, J.E.; Suckling, D.M. Egg Sterilisation of Irradiated Nezara viridula (Hemiptera: Pentatomidae). Insects 2020, 11, 564. https://doi.org/10.3390/insects11090564
Horrocks KJ, Welsh T, Carpenter JE, Suckling DM. Egg Sterilisation of Irradiated Nezara viridula (Hemiptera: Pentatomidae). Insects. 2020; 11(9):564. https://doi.org/10.3390/insects11090564
Chicago/Turabian StyleHorrocks, Kiran Jonathan, Taylor Welsh, Jim E Carpenter, and David Maxwell Suckling. 2020. "Egg Sterilisation of Irradiated Nezara viridula (Hemiptera: Pentatomidae)" Insects 11, no. 9: 564. https://doi.org/10.3390/insects11090564