1. Introduction
Our knowledge of the semiochemistry of the large beetle family Cerambycidae has advanced rapidly over the past 15 years, with several dozen pheromone components with quite varied structures being identified from several hundred species worldwide [
1]. Work has advanced particularly rapidly for cerambycid species in the subfamilies Cerambycinae, Lamiinae, and Spondylidinae, whose adult males produce relatively large quantities of so-called aggregation-sex pheromones [
2], which bring conspecific males and females together on their host plants for mating and reproduction [
3]. A remarkable feature of the chemistry of these compounds is that some structures appear to be highly conserved among both closely related (e.g., congeners) and more distantly related species (e.g., species in different subfamilies), in which the beetles may produce similar or even identical pheromone components [
1]. For example, many cerambycine species use pheromones consisting of 6, 8, or 10 carbon chains with ketone or alcohol groups at C2 and C3, or alcohols in both positions [
1]. Similarly, hydroxyethers such as monochamol (2-[undecyloxy]ethanol), and terpenoid derivatives such as fuscumol ([
E]-6,10-dimethyl- 5,9-undecadien-2-ol) and fuscumol acetate ([
E]-6,10-dimethyl-5,9-undecadien-2-yl acetate) are used as pheromone components by a number of lamiine and spondylidine species [
1]. Additionally, this parsimony in biosynthesis and the use of similar structural motifs as pheromones often extends beyond continental boundaries, with compounds such as 3-hydroxyhexan-2-one being used as pheromone components by cerambycid species endemic to all continents except Antarctica [
3], indicating that this and other shared structures have been conserved within the family for millions of years.
From a practical viewpoint, this parsimony implies that multiple cerambycid species may be attracted to traps baited with single components or blends of related pheromone components [
4,
5,
6,
7,
8,
9,
10]. As such, these pheromones are finding increasing use in the delineation of the geographic ranges of native species [
11], and in surveillance programs for detecting exotic species [
12]. In particular, wood-boring cerambycid beetles are of major importance as potential invaders, because of the ease with which they are transported between continents in wooden products and packing materials by global commerce [
9,
10,
12]. This parsimony also implies that the identification of pheromone components for one cerambycid species may subsequently expedite the identification of pheromones or likely pheromones for related target species [
8,
13,
14].
By contrast, some cerambycid species appear to produce uncommon or even unique pheromone motifs, which may be species-specific or used by a limited number of species. For example, (
Z)-3-decenyl (
E)-2-hexenoate is the male-produced aggregation-sex pheromone of the North American species
Rosalia funebris Motschulsky, and this compound has not attracted any other cerambycid species in field trials in North America, Asia, or Europe [
1]. Similarly, 10-methyldodecanal [
15] and (
Z)-7-hexadecene [
16] have been identified as aggregation-sex pheromone components of the South American species
Eburodacrys vittata (Blanchard) and
Susuacanga octoguttata (Germar), respectively, and these compounds have hitherto not attracted any additional species in field trials in Brazil [
16] or the eastern United States (LMH unpublished data).
However, this apparent species-specificity can be misleading, because pheromones are often blends, in which single components of the blend may have little or no activity. Here, we present evidence that functionalized 10-methyldodecanes, along with the homologous functionalized 11-methyltridecanes, are shared pheromone components of at least eight South American cerambycid species in the subfamily Cerambycinae.
2. Materials and Methods
The eight cerambycid study species included five
Eburodacrys species (tribe Eburiini), two
Compsibidion species (Neoibidionini), and
Tetraopidion mucoriferum (Thomson) (Neoibidionini). To our knowledge, all the species are endemic to South America, with
Eburodacrys assimilis Gounelle and
Eburodacrys lenkoi Napp and Martins only being known from Brazil [
17]. The larvae of
Eburodacrys dubitata White,
Eburodachrys flexuosa Gounelle,
Compsibidion graphicum (Thomson), and
Compsibidion sommeri (Thomson) have been reported to infest several tree species in the family Fabaceae, including species in the genera
Acacia,
Mimosa,
Piptadenia, and
Senegalia [
17]. However, to our knowledge, there is no published information on the biology or hosts of
E. assimilis,
E. lenkoi, and
T. mucoriferum.
2.1. Source of Beetles for Collection of Headspace Volatiles
Adults of both sexes of E. dubitata were obtained from infested twigs of Senegalia polyphylla (DC.) Britton and Rose (Fabaceae) from a forest remnant of Cerrado (Brazilian savanna) located in Valentim Gentil, in the Brazilian state of São Paulo (20°22′19.2″S, 50°04′48.0″W), on 15 November 2015. The twigs were sawn into 30-cm pieces and housed in plastic containers under laboratory conditions (25 ± 1 °C, 60 ± 10% RH, 12 h photophase, and 5000 lux light intensity) in the Laboratory of Chemical Ecology and Insect Behavior, University of São Paulo, Piracicaba (~400 km from VG). Beetles emerged from twigs throughout January and February 2016. Adults of E. assimilis were collected with black light traps deployed near the above-mentioned forest throughout November 2016. Captured beetles were sent overnight by courier to Piracicaba.
For
E. flexuosa and
E. lenkoi, as soon as we found that the adults of both species were attracted by some treatment lures in the bioassays for
E. assimilis (see Results), we deployed an extra set of four traps with the same attractants, to collect live adults of these species. We used cross-vane intercept panel traps (black corrugated plastic) hung from inverted L-shaped frames made from PVC pipe (for details see [
14]). Rainwater was drained from the collection jars suspended below the traps by drilling 2-mm holes in the bottoms of the jars. We coated the surfaces of the traps and the internal surfaces of the collecting jars with an aqueous dispersion of Fluon
® (Insect-a-Slip; Bioquip, Rancho Dominguez, CA). Lures consisted of clear plastic press-seal sachets (polyethylene, 5 × 7.5 cm, 0.05 mm wall thickness; #01-816-1A, Fisher Scientific, Pittsburg, PA, USA), containing a cotton dental roll, loaded with a blend of 50 mg each of racemic 10-methyldodecanal and racemic 11-methyltridecanal, diluted in 900 μL of isopropanol. Lures were hung in the central open slot of traps, which were deployed in a forest remnant of Cerrado in Anhembi (~85 km from Piracicaba; 22°43′04.8″S, 48°10′26.4″W) and checked daily for captured beetles from 11 to 18 December 2018. Beetles were sent to Piracicaba on the day that they were captured.
The first adult of C. sommeri used for aerations was a male caught with a black light trap in Anhembi in 18 December 2018. Another three males and four females were collected in a remnant of the Atlantic Rainforest on the campus of the University of São Paulo, Piracicaba (22°42′43.4″S, 47°37′39.9″W), between 11 October and 18 November 2019. In this case, we used two traps baited with a blend of two synthetic pheromone candidates that were identified from the first specimen, i.e., 10-methyldodecanal and 10-methyldodecanol. The latter compound had been used in field bioassays in Anhembi (see details below), where it did not result in the attraction of any adults of C. sommeri, but it did attract adults of the congener C. graphicum. Consequently, the traps deployed in Piracicaba also made it possible to capture live adults of this species. In addition, we had previously collected two males of C. graphicum on citrus bushes in that location on 15 October 2019, and these were also used for pheromone collection.
Adults of all
Eburodacrys species were sexed based on antennal length; antennae of males are more than twice their body length, whereas antennae of females are slightly longer than the body length [
18]. Sexing of
Compsibidion species was based on the morphology of antennomer III, which is thickened in males [
19]. Males and females of all species were kept separately in plastic cages, with glass vials filled with 10% sugar solution for nourishment, for 48 h before being used for collection of headspace volatiles.
Voucher specimens of all the cerambycid study species have been deposited in the collection of the museum in the Department of Entomology and Acarology (USP/ESALQ), Piracicaba, SP, Brazil.
2.2. Collection of Headspace Volatiles from Beetles
Adult beetles were aerated individually or in groups of two of the same sex in custom-made cylindrical glass chambers (25 cm long × 6 cm i.d.), containing two glass vials with sugar solution. Volatiles emitted by beetles were trapped on 150 mg of 80/100 mesh HayeSep® Q adsorbent (Supelco, Bellefonte, PA, USA) in a glass pipette (8.5 cm long × 0.5 cm i.d.), with the adsorbent held in place with glass wool plugs. Collectors were connected to the outlets of the chambers, with a screw cap fitted with a Teflon ferrule. Charcoal-filtered air was pushed through the chambers at 150 mL/min. Headspace volatiles were collected continuously from beetles for 48 h under the laboratory conditions described above, and collections were made as many as four times from each beetle. Aerations from chambers containing only feeder vials were made in parallel as controls to monitor for system contaminants.
Volatiles were eluted from collectors with three 500-μL aliquots of methylene chloride, into silanized amber glass vials. Each extract was then concentrated to ~500 μL under a gentle flow of N2 and stored at −30 °C until analysis. Overall, aeration extracts were obtained in the following numbers (males/females) from each species: E. assimilis (6/2); E. dubitata (16/9); E. flexuosa (8/4); E. lenkoi (12/5); C. graphicum (8/4); and C. sommeri (11/3). Sex-specific volatile compounds were detected in ~50% of aeration samples from males, but were not detected in any extracts from females.
2.3. Analysis of Extracts of Headspace Volatiles
Extracts of volatiles were initially analyzed in Brazil by gas chromatography with flame ionization detection (GC-FID), or by gas chromatography-mass spectrometry (GC-MS), to confirm the presence of sex-specific compounds. One microliter of extract was injected into a GC-2010 gas chromatograph (Shimadzu Corp., Kyoto, Japan), fitted with an HP5-MS capillary column (30 m × 0.25 mm i.d. × 0.25 µm film; Agilent Technologies, Santa Clara, CA, USA). Injections were made in splitless mode (purge valve off for 1 min), with an injector temperature of 250 °C and helium carrier gas at a linear velocity of 25 cm/s. In the same fashion, injections were made in a Shimadzu QP2010 Ultra GCMS (Shimadzu Corp., Kyoto, Japan), fitted with a nonpolar column (30 m × 0.25 mm × 25 µm film; Rxi-1MS; Restek, Bellefonte, PA, USA). Ion source and quadrupole temperatures were set at 250 °C. Mass spectra were recorded in electron impact mode (70 eV) from m/z 35–280. The GC oven was programmed from 35 °C for 1 min, increased to 40 °C at 2 °C/min, hold 1 min, and increased to 250 °C at 10 °C/min, hold 10 min. Representative extracts that contained detectable amounts of sex-specific compounds were sent to the University of California, Riverside (UCR), for identification of the sex-specific compounds. At UCR, extracts were reanalyzed with an Agilent 7820A GC, coupled to a 5977E mass selective detector. The GC was equipped with an HP-5 column (same dimensions as above), and samples were injected in splitless mode. Helium carrier gas was used, with an oven temperature program of 40 °C/5 min, 10 °C/min to 280 °C (hold 10 min). The injector, ion source, and quadrupole temperatures were 250, 230, and 150 °C respectively. Mass spectra were obtained with electron impact ionization (70 eV), scanning a mass range from 40–400 amu. Retention indices were calculated by comparison with a blend of straight-chain alkane standards.
2.4. Synthetic Chemicals
10-Methyldodecanol and 10-methydodecanal were synthesized as described in [
15]. The synthesis of 11-methyltridecanal was carried out as shown in
Figure 1, and as described in detail in
Figure S1.
2.5. Field Bioassays of Pheromone Candidates
We field-tested the synthetic version of the male-produced compound from
E. dubitata (i.e., 11-methyltridecanal) in Valentim Gentil, from 5 December 2016 to 8 January 2017. We used cross-vane panel traps as described above, except that the collection jars were modified to hold 300 mL of an aqueous solution of dish soap and NaCl to kill and preserve the captured beetles. The lures (press-seal sachets with a cotton dental roll) were loaded with 50 mg of racemic 11-methyltridecanal, diluted in 950 µL of isopropanol. Control lures contained 1 mL of neat isopropanol. Traps were spaced ~15 m apart in four pairs (blocks), which were ~30 m apart. Previous studies have demonstrated that this is an ample distance between traps to prevent interference between treatments within a trap transect [
20]. Pheromone and control treatments were assigned randomly to traps, and each pair contained one trap with each treatment.
Trap catches were counted every 2–3 d, at which time the preservative solution was replaced and treatment traps were switched in position within the block to control for positional bias. Lures were replaced every 15 d.
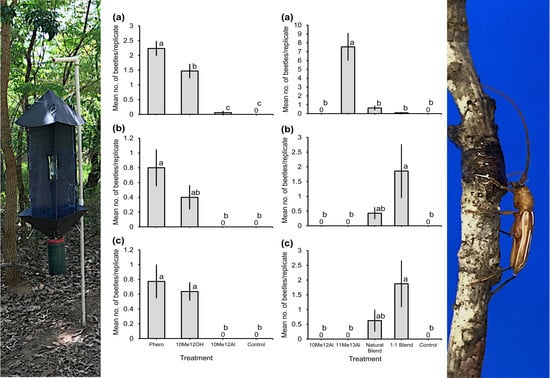
Field bioassays with E. assimilis were carried out in Valentim Gentil (from 28 November to 24 December 2018) and Anhembi (from 7 November 2018 to 5 January 2019). The lures contained 1 mL of a solution of synthetic pheromone candidates in isopropanol, with the following treatments: 1) racemic 10-methyldodecanal (50 mg); 2) racemic 11-methyltridecanal (50 mg); 3) natural blend of racemic 10-methyldodecanal (18 mg) and 11-methyltridecanal (50 mg), which mimics the ratio produced by adult males of E. assimilis; 4) 1:1 blend of these aldehydes (50 mg each); and 5) control (1 mL of isopropanol). The pheromone and control treatments were assigned randomly to traps within three (Valentim Gentil) and four (Anhembi) blocks, and each block contained one trap with each treatment. Traps were spaced ~15 m apart and blocks were ~30 m from each other. Traps were checked for beetles every 2–3 d in Valentim Gentil and every 15 d in Anhembi, following the procedures described above.
For the Compsibidion species, we had previously conducted two bioassays testing synthetic 10-methyldodecanol, before we had any evidence that Compsibidion species might produce this compound. This alcohol was originally identified from a North American cerambycid species (also tribe Neoibidionini; LMH and JGM unpublished data). Therefore, the bioassays in Brazil were intended to field screen this compound, in case any South American species might be attracted to it, as a lead to the identification of their pheromones. The compound was tested against controls in field bioassays in Anhembi from 24 November to 5 January 2019, using lures loaded with 50 mg of racemic 10-methyldodecanol, as described above. Control lures contained 1 mL of isopropanol. A total of two pairs of traps, each one containing one pheromone and one control treatment, were used. Traps were checked for beetles every ~15 d, as described above.
After the identification of male-specific volatiles from C. sommeri and C. graphicum, we followed up with bioassays testing 10-methyldodecanal and 10-methyldodecanol, individually and in blends. The treatment lures were: 1) a blend of 10-methyldodecanal (2.5 mg) and 10-methyldodecanol (50 mg), similar to the ratios produced by the beetles; 2) 10-methyldodecanol (50 mg); 3) 10-methyldodecanal (2.5 mg); and 4) control (1 mL of isopropanol). Treatments were assigned randomly to traps within six blocks, and each block contained representatives of all treatments. Traps were hung from tree branches at ~3 m from the ground and spaced 20 m apart, and blocks were 40 m from each other. Trap catches were tallied every 2–3 d from 12 November to 28 December 2019, following the same procedures used in the other bioassays.
2.6. Statistical Analysis
Differences between treatment means were tested separately for species represented by at least eight specimens per bioassay, using either the two-tailed Exact Binomial test (if there were only two treatments; [
21]) or the nonparametric Friedman’s test (for greater numbers of treatments; PROC FREQ, option CMH; [
22]), because field data violated assumptions of ANOVA [
23]. Replicates were defined by spatial (block) and temporal (collection date) data. We included in each analysis only replicates that contained a minimum number of specimens, which ranged from 1 to 3 depending on species, in order to ensure a minimum number of replicates for a robust analysis (N ≥ 7 replicates). In recognition of the multiple statistical tests of treatment effects, significance levels were adjusted in the bioassays for
E. assimilis in Valentim Gentil (α = 0.025; N = two analyses) and Anhembi (α = 0.017; N = three analyses), and in the bioassays for
Compsibidion spp. (α = 0.017), according to the Bonferroni procedure [
24]. Pairs of means were compared using the REGWQ multiple range test, which controls the Type I experiment-wise error rate [
22]. The binomial test was performed with a spreadsheet available at
http://www.biostathandbook.com/exactgof.html (accessed 31 January 2020).
The sex ratio of beetles captured by traps with the optimal attractant was compared to a nominal proportion of 0.5 with 95% Clopper–Pearson exact confidence intervals at 5% probability [
25].
4. Discussion
Recently, 10-methyldodecanal has been been identified as an attractant pheromone for the South American cerambycid beetle
E. vittata, and we initially thought that this structural motif might be species-specific [
15]. However, the results presented here demonstrate that this is not the case, because this compound and the analogous alcohol, and the one carbon homolog 11-methyltridecanal, constitute aggregation-sex pheromone components of seven additional sympatric cerambycid species.
We first analyzed the pheromone chemistry of the congener
E. dubitata, whose adult males sex-specifically produce 11-methyltridecanal as a single component, which attracted conspecific adults of both sexes in field bioassays. Concomitantly, we found that males of
E. assimilis produce a blend of 10-methyldodecanal and 11-methyltridecanal. However, field trials with these compounds as single components and in blends failed to attract
E. assimilis, but serendipitously, they offered new insights into the likely pheromone chemistry of two congeners,
E. flexuosa and
E. lenkoi. Both sexes of these species were strongly attracted to a 1:1 blend of 10-methyldodecanal and 11-methyltridecanal. We subsequently found that males of both species produced approximately equal amounts of these two homologous aldehydes. In addition, these bioassays confirmed that 10-methyldodecanal as a single component only attracts
E. vittata, as previously found [
15], whereas 11-methyltridecanal as a single component was specifically attractive to
E. dubitata.
We also showed that males of C. graphicum and C. sommeri sex-specifically produce a blend of 10-methyldodecanol, with much smaller but crucial amounts of 10-methyldodecanal. In the field, traps baited with 10-methyldodecanol did attract adults of both species, but the attraction of C. graphicum increased significantly when small amounts of 10-methyldodecanal were included in the lure. During these field trials, adults of yet another species, T. mucoriferum, were significantly attracted to treatments containing 10-methyldodecanol, offering good circumstantial evidence that this compound might also be a pheromone component of this species as well.
Taken together, these results represent another demonstration that, analogous to other insect taxa, sympatric cerambycid species evolve specific pheromone channels from a basic pheromone motif, by varying the combinations and ratios of components, with either strong synergism between components, or conversely, strong antagonism by one or more components that limit the cross-attraction of species which share the main pheromone component. Further complexity in the signal can be created by using components of different chain lengths, and/or components with the same carbon skeleton, but different functional groups. In addition, another layer of signal complexity may be possible, because each of the four compounds described above can exist as (R)- or (S)-enantiomers. In fact, this may explain the lack of attraction of E. assimilis in field trials, despite the clear analytical evidence that it produces a blend of 10-methyldodecanal and 11-methyltridecanal. That is, in field trials to date, we have only tested racemic compounds, and if adults of E. assimilis are strongly inhibited by one of the enantiomers of either one of these compounds, this could explain the complete lack of attraction to lures formulated with racemic compounds.
To date, we have not been able to resolve the enantiomers of either the alcohols or the aldehydes by chromatographic methods, in part due to the large separation between the functional group and the methyl branch. Thus, it will likely be necessary to estimate which enantiomer of each compound is produced by each species through bioassays of the enantiomers, or possibly non-racemic mixtures of the two enantiomers of each component. Such tests may prove very revealing, given several recent examples demonstrating enantiomeric discrimination, and synergism between enantiomers, for several cerambycid species [
14,
27,
28].
Our results also provide another example of the frequent strong conservation of pheromone motifs among congeners and even more distantly related cerambycid species in different tribes. The data reported here show that the 10-methyldodecyl and 11-methyltridecyl motifs are conserved within a number of South American species in the tribes Eburiini and Neoibidionini. However, we also have evidence that the 10-methyldodecyl skeleton may be used by some North American species in the tribe Neoibidionini as well (LMH unpublished data). Given the large numbers of species in these tribes [
17], we fully expect that additional species will be found using these pheromone components. We plan to address the role of enantiomers in the pheromone blends of these and related species in ongoing studies.
The results described here also have important implications for insect surveillance efforts worldwide, whose goal is the detection of new incursions of invasive species. In particular, our results, along with previously published work [
15], suggest that 10-methyldodecanal, 11-methyltridecanal, and their corresponding alcohols represent a pheromone motif that is used by cerambycine species in several different genera in at least two different tribes, on at least two different continents. Furthermore, because the chemistry of these pheromones is markedly different than the known pheromones of other cerambycid species, it seems highly likely that these compounds could be incorporated into generic lures containing pheromones of a number of species, with minimal chance of inhibiting attraction of other species. Thus, we anticipate that these new pheromones will be of substantial value and use to regulatory agencies charged with the detection and monitoring of invasive insect pests.