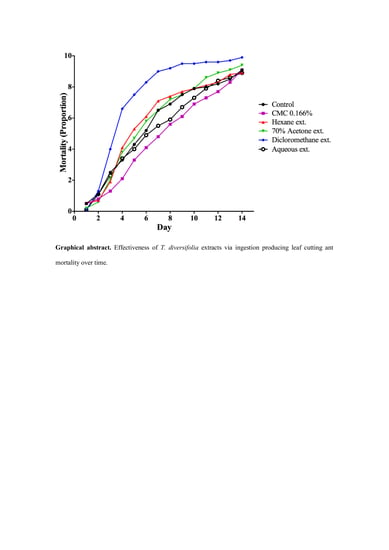
Insecticidal and Cholinesterase Activity of Dichloromethane Extracts of Tithonia diversifolia on Atta cephalotes Worker Ants (Formicidae: Myrmicinae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Atta cephalotes Colonies
2.2. Plant Material
2.3. Extraction and Fractionation
2.4. Toxicity of DMSO and CMC on A. cephalotes Worker Ants
2.5. Experimental Design and Statistical Analysis
2.6. Cholinesterase Activity
3. Results
3.1. Toxicity of DMSO and CMC on A. cephalotes Worker Ants
3.2. Cholinesterase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Erthal, M.; Silva, C.P.; Samuels, R.I. Digestive enzymes in larvae of the leaf cutting ant, Acromyrmex subterraneus (Hymenoptera: Formicidae: Attini). J. Insect. Physiol. 2007, 53, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Mueller, U.G.; Rabeling, C. A breakthrough innovation in animal evolution. PNAS 2008, 105, 5287–5288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauh, V.A.; Perera, F.P.; Horton, M.K.; Whyatt, R.M.; Bansal, R.; Hao, X.; Liu, J.; Barr, D.B.; Slotkin, T.A.; Peterson, B.S. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. PNAS 2012, 109, 7871–7876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, A.; Zanetti, R.; Dos Santos, J.C.; Biagiotti, G.; Evangelista, A.L.; Serrão, J.E.; Zanuncio, J.C. Persistence of fipronil residues in eucalyptus seedlings and its concentration in the insecticide solution after treatment in the nursery. Environ. Monit. Assess. 2016, 188, 314. [Google Scholar] [CrossRef]
- Lobo-Echeverri, T.; Salazar, L.C.; Hernández, A.; Ortiz-Reyes, A. Effects of Capsicum baccatum and C. frutescens against Atta cephalotes (Hymenoptera: Formicidae) and the symbiotic fungus Leucoagaricus gongylophorus. Rev. Col. Entomol. 2016, 42, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.; Enimar, A.; Magalhães, J.; Leivas, C.; Centenaro, E. Growth of symbiont fungi of cutter ants of the genus Acromyrmex in means of culture with different extracts. Cienc. Rural 2006, 36, 725–730. [Google Scholar]
- Giraldo, C. Efecto del botón de oro Tithonia diversifolia sobre la herbivoría de hormiga arriera Atta cephalotes en una plantación de arboloco Montanoa quadrangularis. In Graduate Monograph; Universidad del Valle: Cali, Colombia, 2005. [Google Scholar]
- Rodríguez, J.; Calle, Z.; Montoya-Lerma, J. Herbivoría de Atta cephalotes (Hymenoptera: Myrmicinae) sobre tres sustratos vegetales. Rev. Col. Entomol. 2008, 34, 156–162. [Google Scholar]
- Valderrama-Eslava, E.; Montoya-Lerma, J.; Giraldo, C. Enforced herbivory on Canavalia ensiformis and Tithonia diversifolia and its effects on leaf-cutting ants, Atta cephalotes. J. Appl. Entomol. 2009, 133, 689–694. [Google Scholar] [CrossRef]
- Castaño-Quintana, K.; Montoya-Lerma, J.; Giraldo, C. Toxicity of foliage extracts of Tithonia diversifolia (Asteraceae) on Atta cephalotes (Hymenoptera: Myrmicinae) workers. Ind. Crop. Prod. 2013, 44, 391–395. [Google Scholar] [CrossRef]
- Rodríguez, J.; Montoya-Lerma, J.; Calle, Z. Effect of Tithonia diversifolia Mulch on Atta cephalotes (Hymenoptera: Formicidae) nests. J. Insect. Sci. 2015, 15, 32. [Google Scholar] [CrossRef] [Green Version]
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Menichini, F. Natural products and their derivatives as cholinesterase inhibitors in the treatment of neurodegenerative disorders: An update. Curr. Med. Chem. 2008, 15, 1209–1228. [Google Scholar] [CrossRef] [PubMed]
- Valderrama-Eslava, E.; Giraldo, C.; Montoya-Lerma, J.; Armbrecht, I.; Calle, Z. Guía para el establecimiento y manejo de colonias artificiales de Hormiga arriera Atta cephalotes (Hymenoptera: Myrmicinae). Bol. Mus. Entomol. Univ. 2006, 7, 9–16. [Google Scholar]
- Bueno, F.C.; Godoy, M.P.; Leite, A.C.; Bueno, O.C.; Pagnocca, F.C.; Fernandes, J.B.; Hebling, M.; Bacci, M.J.A., Jr.; Vieira, P.C.; Silva, M.F.G.F. Toxicity of Cedrela fissilis to Atta sexdens rubropilosa (Hymenoptera: Formicidae) and its symbiotic fungus. Sociobiology 2005, 45, 389–399. [Google Scholar]
- Core, R. Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Bilder, C.R.; Loughin, T.M. Analysis of Categorical Data with R.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2014; p. 547. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Mello, M.O.; Silva-Filho, M.C. Plant-insect interactions: An evolutionary arms race between two distinct defense mechanisms. Braz. J. Plant Physiol. 2002, 14, 71–81. [Google Scholar] [CrossRef]
- Cheng, A.X.; Lou, Y.G.; Mao, Y.B.; Lu, S.; Wang, L.J.; Chen, X.Y. Plant terpenoids: Biosynthesis and Ecological Functions. J. Integr. Plant Biol. 2007, 49, 179–186. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Theis, N.; Lerdau, M. The evolution of function in plant secondary metabolites. Int. J. Plant Sci. 2003, 164, 93–102. [Google Scholar] [CrossRef]
- Després, L.; David, J.P.; Gallet, C. The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol. Evol. 2007, 22, 298–307. [Google Scholar] [CrossRef]
- Ambrósio, S.R.; Oki, Y.; Heleno, V.C.; Chaves, J.S.; Nascimento, P.G.; Lichston, J.E.; Constantino, M.G.; Varanda, E.M.; Da Costa, F.B. Constituents of glandular trichomes of Tithonia diversifolia: Relationships to herbivory and antifeedant activity. Phytochemistry 2008, 69, 2052–2060. [Google Scholar] [CrossRef]
- Chagas-Paula, D.; Oliveira, R.; Rocha, B.; Da Costa, F. Ethnobotany, Chemistry, and Biological activities of the genus Tithonia (Asteraceae). Chem. Biodivers. 2012, 9, 210–235. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Yokosuka, A.; Kobatashi, R.; Jitsuno, M.; Kando, H.; Nosaka, K.; Ishii, H.; Yamori, T.; Mimaki, Y. Sesquiterpenoids and flavonoids from the aerial parts of Tithonia diversifolia and their cytotoxic activity. Chem. Pharm. Bull. 2007, 55, 1240–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouberte, M.Y.; Krohn, K.; Hussain, H.; Dongo, E.; Schulz, B.; Hu, Q. Tithoniaquinone A and tithoniamide B: A new anthraquinone and a new ceramide from leaves of Tithonia diversifolia. Z. Naturforsch. B 2006, 61, 78–82. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Tempora, M.M.; Rideout, J.A. Terpenoids from Tithonia diversifolia. J. Res. Sci. Comp. Engin. 2007, 4, 1–7. [Google Scholar] [CrossRef]
- Serna, F.; Correa, J. Lycopersicon esculentum tomato leaf extracts as phago-inhibitors of Atta cephalotes leaf-cutter ants. Agron. Colomb. 2003, 21, 142–153. [Google Scholar]
- Eigenbrode, S.D. Effects of plant epicuticular lipids on insect herbivores. Ann. Rev. Entomol. 1995, 40, 171–194. [Google Scholar] [CrossRef]
- Terrance, H.D.; Wiemer, D.F. Ant-repellent terpenoids from Melampodium divaricatum. Phytochemistry 1985, 24, 1197–1198. [Google Scholar]
- Okunade, A.L.; Wiemer, D.F. Ant-repellent sesquiterpene lactones from Eupatorium quadrangularae. Phytochemistry 1985, 24, 1199–1201. [Google Scholar] [CrossRef]
- Oyedokun, A.V.; Anikwe, J.C.; Okelana, F.A.; Mokwunye, I.U.; Azeez, O.M. Pesticidal efficacy of three tropical herbal plants’ leaf extracts against Macrotermes bellicosus, an emerging pest of cocoa, Theobroma cacao L. J. Biopest. 2011, 4, 131–137. [Google Scholar]
- Adedire, C.O.; Akinneye, J.O. Biological activity of tree marigold, Tithonia diversifolia, on cowpea seed bruchid, Callosobruchus maculatus (Coleoptera: Bruchidae). Ann. Appl. Biol. 2004, 144, 185–189. [Google Scholar] [CrossRef]
- Oyewole, I.O.; Adeoye, G.O.; Anyasor, G.; Obansa, J.A. Anti-malarial and repellent activities of Tithonia diversifolia (Hemsl.) leaf extracts. J. Med. Plant Res. 2008, 2, 171–175. [Google Scholar]
- Dai, D.N.; Thang, T.D.; Ogunmoye, A.; Eresanya, O.I.; Ogunwande, I.A. Chemical constituents of essential oils from the leaves of Tithonia diversifolia, Houttuynia cordata and Asarum glabrum grown in Vietnam. Am. J. Essent. Oil. Nat. Prod. 2015, 2, 17–21. [Google Scholar]
- Miranda, M.A.; Varela, R.M.; Torres, A.; Molinillo, J.M.; Gualtieri, S.C.; Macías, F.A. Phytotoxins from Tithonia diversifolia. J. Nat. Prod. 2015, 78, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Gillij, Y.G.; Gleise, R.M.; Zygadlo, J.A. Mosquito repellent activity of essential oils of aromatic plants growing in Argentina. Bioresour. Technol. 2008, 99, 2507–2515. [Google Scholar] [CrossRef] [PubMed]
- Céspedes, C.L.; Alarcon, J.E.; Aqueveque, P.; Seigler, D.S.; Kubo, I. In the search for new secondary metabolites with biopesticidal properties. Isr. J. Plant Sci. 2015, 62, 216–228. [Google Scholar] [CrossRef]
- Ryan, M.F.; Byrne, O. Plant-insect coevolution and inhibition of acetylcholinesterase. J. Chem. Ecol. 1988, 14, 1965–1975. [Google Scholar] [CrossRef]
- Radic, Z.; Pickering, N.A.; Vellom, D.C.; Camp, S.; Taylor, P. Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase inhibitors. Biochemistry 1993, 32, 12074–12084. [Google Scholar] [CrossRef]
- Céspedes, C.L.; Ávila, J.R.; Marin, J.C.; Domínguez, L.M.; Torres, P.; Aranda, E. Natural compounds as antioxidant and molting inhibitors can play a role as a model for search of new botanical pesticides. In Naturally Occurring Bioactive Compounds: Advances in Phytomedicine Series; Rai, M., Carpinella, M.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–127. [Google Scholar]
- Fournier, D.; Bride, J.M.; Hoffmann, F.; Karch, F. Acetylcholinesterase: Two types of modifications confer resistance to insecticide. J. Biol. Chem. 1992, 267, 14270–14274. [Google Scholar]
- Miyazawa, M.; Watanabe, H.; Kameoka, H. Inhibition of Acetylcholinesterase Activity by Monoterpenoids with a p-Menthane Skeleton. J. Agr. Food Chem. 1997, 45, 677–679. [Google Scholar] [CrossRef]
- Céspedes, C.L.; Muñoz, E.; Salazar, J.R.; Yamaguchi, L.; Werner, E.; Alarcon, J.; Kubo, I. Inhibition of cholinesterase activity by extracts, fractions and compounds from Calceolaria talcana and C. integrifolia (Calceolariaceae: Scrophulariaceae). Food Chem. Toxicol. 2013, 62, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Céspedes, C.L.; Salazar, J.R.; Ariza-Castolo, A.; Yamaguchi, L.; Avila, J.G.; Aqueveque, P.; Kubo, I.; Alarcón, J. Biopesticides from plants: Calceolaria integrifolia s.l. Environ. Res. 2014, 132, 391–406. [Google Scholar] [CrossRef] [PubMed]


| Treatment | DMSO and CMC | T. diversifolia Extracts at 1000 ppm | Comparison Dichloromethane Extracts | ||
|---|---|---|---|---|---|
| Ingestion | Contact | Ingestion | Contact | Ingestion | |
| T1 | Diet | Diet | Diet | Diet | Diet |
| T2 | Diet + CMC 0.25% | CMC 0.125% | Diet + C1 | C2 | Diet + C1 |
| T3 | Diet + CMC 0.5% | CMC 0.25% | Diet + Hexane extract in C1 | Hexane extract in C2 | Diet + Dichloromethane extract (250 ppm) in C1 |
| T4 | Diet + DMSO 0.5% | DMSO 0.5% | Diet + Acetone 70% extract in C1 | Acetone 70% extract in C2 | Diet + Dichloromethane (500 ppm) in C1 |
| T5 | Diet + DMSO 1% | DMSO 1% | Diet + Dichloromethane extract in C1 | Dichloromethane extract in C2 | − |
| T6 | Diet | Diet | Diet | Diet | Diet |
| Extract/Fraction | Weight (mg) | AChE IC50 (μg/mL) | BuChE IC50 (μg/mL) |
|---|---|---|---|
| Hexane extract | 19.13 × 103 | 260.57 ± 0.001 | >500 * |
| 70% Acetone extract | 101.10 × 103 | 109.2 ± 12.18 | 60.6 ± 12.17 |
| Dichloromethane extract | 10.60 × 103 | 73.9 ± 11.06 | >500 * |
| Ethyl acetate extract | 2.05 × 103 | 65.6 ± 9.06 | >500 * |
| Butanol extract | 5.25 × 103 | 105.0 ± 18.13 | >500 * |
| Aqueous residue extract | 42.57 × 103 | 130.5 ± 12.23 | >500 * |
| Td 2.1.1 fraction | 45.1 | >500 * | >500 * |
| Td 2.1.2 fraction | 936.1 | 144.5 ± 0.02 | >500 * |
| Td 2.1.3 fraction | 784.5 | 117.72 ± 0.005 | 314.6 ± 0.009 |
| Td 2.1.4 fraction | 2783.5 | 155.64 ± 0.005 | >500 * |
| Td 2.1.5 fraction | 776.4 | 186.77 ± 0.003 | >500 * |
| Td 2.1.6 fraction | 1893.7 | 119.0 ± 0.02 | >500 * |
| Galantamine | 0.54 μM ± 0.7 | 8.80 μM ± 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantoja-Pulido, K.D.; Rodríguez, J.; Isaza-Martínez, J.H.; Gutiérrez-Cabrera, M.; Colmenares-Dulcey, A.J.; Montoya-Lerma, J. Insecticidal and Cholinesterase Activity of Dichloromethane Extracts of Tithonia diversifolia on Atta cephalotes Worker Ants (Formicidae: Myrmicinae). Insects 2020, 11, 180. https://doi.org/10.3390/insects11030180
Pantoja-Pulido KD, Rodríguez J, Isaza-Martínez JH, Gutiérrez-Cabrera M, Colmenares-Dulcey AJ, Montoya-Lerma J. Insecticidal and Cholinesterase Activity of Dichloromethane Extracts of Tithonia diversifolia on Atta cephalotes Worker Ants (Formicidae: Myrmicinae). Insects. 2020; 11(3):180. https://doi.org/10.3390/insects11030180
Chicago/Turabian StylePantoja-Pulido, Kriss D., Jonathan Rodríguez, José H. Isaza-Martínez, Margarita Gutiérrez-Cabrera, Ana J. Colmenares-Dulcey, and James Montoya-Lerma. 2020. "Insecticidal and Cholinesterase Activity of Dichloromethane Extracts of Tithonia diversifolia on Atta cephalotes Worker Ants (Formicidae: Myrmicinae)" Insects 11, no. 3: 180. https://doi.org/10.3390/insects11030180