Combined Effects of Mating Disruption, Insecticides, and the Sterile Insect Technique on Cydia pomonella in New Zealand
Abstract
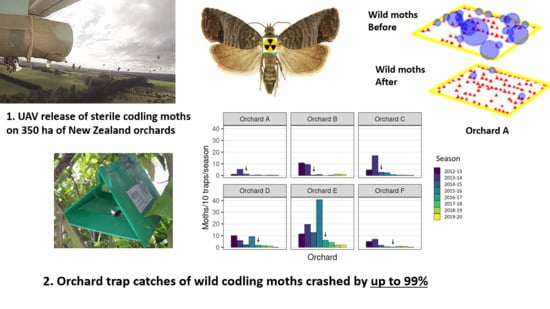
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Orchards
2.2. Sterile Insect Technique
2.2.1. Insect Rearing, Shipment, and Quality Control
2.2.2. Quality Control Testing of Insects Released from Unmanned Aerial Vehicle
2.2.3. Field Release of Insects
2.3. Monitoring Adult Codling Moths
2.4. Mating Disruption
2.5. Insecticides
2.6. Fruit Damage Assessments
2.7. Data Analysis
3. Results
3.1. Quality Control of Long-Distance Shipped Insects
3.2. Quality Control of Insects Released from UAV Device
3.3. Field Trials
4. Discussion
4.1. Wild Moth Trends
4.2. Sterile Moth Release and Recapture
4.3. Future Prospects of an Area-Wide Program in NZ
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- CABI. Cydia Pomonella; CABI: Wallingford, UK, 2019. [Google Scholar]
- Miller, D. Report of the seventh science congress. In Historical Review of New Zealand Entomology; Black, M.A., Ed.; Royal Society of New Zealand: Wellington, New Zealand, 1953; pp. 80–86. [Google Scholar]
- Woods, B.; Thwaite, G.; Monzu, N.; Portman, T.; Power, G.; Davis, P. The history of codling moth eradication from WA. Good Fruit Veg. 2001, 12, 79–81. [Google Scholar]
- Welter, S.C.; Varela, L.; Freeman, R. Codling moth resistance to azinphosmethyl in California. Resist. Pest Manag. 1991, 3, 12. [Google Scholar]
- Ioriatti, C.; Bouvier, J.C.; Butturini, A.; Cornale, R.; Tiso, R. Codling moth: The current status of insecticide resistance in the major pipfruit growing regions in Italy. Inf. Fitopatol. 2003, 53, 53–59. [Google Scholar]
- Suckling, D.M.; Walker, J.T.S.; Wearing, C.H. Ecological impact of three pest management systems in New Zealand apple orchards. Agric. Ecosyst. Environ. 1999, 73, 129–140. [Google Scholar] [CrossRef]
- Wearing, H. Farewell Silent Spring: The New Zealand Apple Story; The New Zealand Plant Protection Society: Auckland, New Zealand, 2019; p. 278. [Google Scholar]
- Morgan, C.V.G.; Gaunce, A.P.; Jong, C. Control of codling moth larvae in harvested apples by methyl bromide fumigation and cold storage. Can. Entomol. 1974, 106, 917–920. [Google Scholar] [CrossRef]
- Witzgall, P.; Stelinski, L.; Gut, L.; Thomson, D. Codling moth management and chemical ecology. Annu. Rev. Entomol. 2008, 53, 503–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suckling, D.M.; El-Sayed, A.M.; Walker, J.T.S. Regulatory innovation, mating disruption and 4-PlayTM in New Zealand. J. Chem. Ecol. 2016, 42, 584–589. [Google Scholar] [CrossRef]
- Walker, J.T.S.; Suckling, D.M.; Wearing, C.H. Past, present, and future of integrated control of apple pests: The New Zealand experience. Annu. Rev. Entomol. 2017, 62, 231–248. [Google Scholar] [CrossRef]
- Wearing, C.H.; Charles, J.G. Cydia pomonella (L), codling moth (Lepidoptera: Tortricidae). In A Review of Biological Control of Invertebrate Pests and Weeds in New Zealand 1874–1987; Cameron, P.J., Hill, R.L., Bain, J., Thomas, W.P., Eds.; CAB International: Wallingford, UK, 1989; pp. 161–169. [Google Scholar]
- Charles, J.G.; Sandanayaka, W.R.M.; Walker, J.T.S.; Shaw, P.W.; Chhagan, A.; Cole, L.M.; Colhoun, K.; Davis, V.A.; Wallis, D.R. Establishment and seasonal activity in New Zealand of Mastrus ridens, a gregarious ectoparasitoid of codling moth Cydia pomonella. Biocontrol 2019, 64, 291–301. [Google Scholar] [CrossRef]
- Suckling, D.M.; Gibb, A.R.; Burnip, G.M.; Delury, N.C. Can parasitoid sex pheromones help in insect biocontrol? A case study of codling moth (Lepidoptera: Tortricidae) and its parasitoid Ascogaster quadridentata (Hymenoptera: Braconidae). Environ. Entomol. 2002, 31, 947–952. [Google Scholar] [CrossRef]
- Horner, R.; Paterson, G.; Walker, J.T.S.; Perry, G.L.W.; Jaksons, R.; Suckling, D.M. Will peri-urban Cydia pomonella (Lepidoptera: Tortricidae) challenge local eradication? Insects 2020, 11, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anonymous. Fresh Facts. 2019. Available online: https://www.freshfacts.co.nz/files/freshfacts-2019.pdf (accessed on 20 July 2020).
- New Zealand Apples and Pears Inc. Pipfruit Industry Statistical Annual 2017 (year to Dec. 2018); Apples and Pears Inc.: Hastings, New Zealand, 2018; Available online: https://www.applesandpears.nz/ (accessed on 20 July 2020).
- EPA. Methyl Bromide Fumigations: Post-Reassessment Guidance for Fumigators; Environmental Protection Authority: Wellington, New Zealand, 2011.
- Goldson, S.L.; Bourdôt, G.W.; Brockerhoff, E.G.; Byrom, A.E.; Clout, M.N.; McGlone, M.S.; Nelson, W.A.; Popay, A.J.; Suckling, D.M.; Templeton, M.D. New Zealand pest management: Current and future challenges. J. R. Soc. N. Z. 2015. [Google Scholar] [CrossRef]
- Lo, P.L.; Walker, J.T.S.; Horner, R.M.; Hedderley, D.I. Development of multiple species disruption to control codling moth and leafrollers (Lepidoptera: Tortricidae). N. Z. Plant. Prot. 2013, 67, 264–269. [Google Scholar] [CrossRef]
- Suckling, D.M.; Tobin, P.C.; McCullough, D.G.; Herms, D. Combining tactics to exploit Allee effects for eradication of alien insect populations. J. Econ. Entomol. 2012, 105, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knipling, E.F. The basic principles of insect population suppression and management. In Agricultural Handbook; Science and Education Administration; United States Department of Agriculture: Washington, DC, USA, 1979; p. 659. [Google Scholar]
- Vreysen, M.J.B.; Carpenter, J.E.; Marec, F. Improvement of the sterile insect technique for codling moth Cydia pomonella (Linnaeus) (Lepidoptera Tortricidae) to facilitate expansion of field application. J. Appl. Entomol. 2010, 134, 165–181. [Google Scholar] [CrossRef]
- Simmons, G.; Bloem, K.; Bloem, S.; Carpenter, J.; Suckling, D. Impact of moth suppression/eradication programmes using the sterile insect technique or inherited sterility in Sterile Insect Technique. In Principles and Practice in Area-Wide Integrated Pest Management, 2nd ed.; Dyck, A., Hendrichs, J., Robinson, A., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 1007–1050. [Google Scholar]
- Proverbs, M.D.; Newton, J.R.; Logan, D.M. Suppression of codling moth, Laspeyresia pomonella (lepidoptera: Olethreutidae), by release of sterile and partially sterile moths. Can. Entomol. 1978, 110, 1095–1102. [Google Scholar] [CrossRef]
- Proverbs, M.D.; Newton, J.R. Some effects of gamma radiation on the reproductive potential of the codling moth, Carpocapsa pomonella (L.) (Lepidoptera: Olethreutidae). Can. Entomol. 1962, 94, 1162–1170. [Google Scholar] [CrossRef]
- Proverbs, M.D. The sterile male technique and its possible use for codling moth eradication. Can. Entomol. 1964, 96, 143. [Google Scholar] [CrossRef]
- Proverbs, M.D.; Newton, J.R.; Campbell, C.J. Codling moth: A pilot program of control by sterile insect release in British Columbia. Can. Entomol. 1982, 114, 363–376. [Google Scholar] [CrossRef]
- Butt, B.A.; White, L.D.; Moffitt, H.R.; Hathaway, D.O.; Schoenleber, L.G. Integration of sanitation, insecticides, and sterile moth releases for suppression of populations of codling moths in the Wenas Valley of Washington. Environ. Entomol. 1973, 2, 208–212. [Google Scholar] [CrossRef]
- White, L.D.; Hutt, R.B.; Butt, B.A. Field dispersal of laboratory-reared fertile female codling moths and population suppression by release of sterile males. Environ. Entomol. 1973, 2, 66–69. [Google Scholar] [CrossRef] [Green Version]
- Bloem, S.; Carpenter, J.E.; McCluskey, A.; Fugger, R.; Arthur, S.; Wood, S. Suppression of the codling moth Cydia pomonella in British Columbia, Canada using an area-wide integrated approach with an SIT component. In Area-Wide Control of Insect Pests: From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 591–602. [Google Scholar]
- Thistlewood, H.; Judd, G.J. Twenty-five years of research experience with the sterile insect technique and area-wide management of codling moth, Cydia pomonella (L.), in Canada. Insects 2019, 10, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taret, G.; Sevilla, M.; Wornoayporn, V.; Islam, A.; Ahmad, S.; Caceres, C.; Robinson, A.S.; Vreysen, M.J.B. Mating compatibility among populations of codling moth Cydia pomonella Linnaeus (Lepidoptera: Tortricidae) from different geographic origins. J. Appl. Entomol. 2010, 134, 207–215. [Google Scholar] [CrossRef]
- Blomefield, T.; Carpenter, J.E.; Vreysen, M.J.B. Quality of mass-reared codling moths (Lepidoptera: Tortricidae) after long-distance transportation: 1. Logistics of shipping procedures and quality parameters as measured in the laboratory. J. Econ. Entomol. 2011, 104, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.; Hofmeyr, J.; Groenewald, S.; Conlong, D.; Wohlfarter, M. The sterile insect technique in agricultural crops in South Africa: A metamorphosis but will it fly? Afr. Entomol. 2015, 23, 1–18. [Google Scholar] [CrossRef]
- FAO. International Standards for Phytosanitary Measures ISPM 22: Requirements for the Establishment of Areas of Low Pest Prevalence; [Accessed on 20 July 2018]; Food and Agricultural Organisation of the United Nations: Rome, Italy, 1995. [Google Scholar]
- FAO. International Standards for Phytosanitary Measures ISPM 4: Requirements for the Establishment of Pest Free Areas; FAO: Rome, Italy, 2017. [Google Scholar]
- FAO. International Standards for Phytosanitary Measures ISPM 5: Glossary of Phytosanitary Terms; FAO: Rome, Italy, 2019. [Google Scholar]
- Paterson, G.; Perry, G.L.W.; Walker, J.T.S.; Suckling, D.M. Peri-urban community attitudes towards codling moth trapping and suppression using the sterile insect technique in New Zealand. Insects 2019, 10, 335. [Google Scholar] [CrossRef] [Green Version]
- Bloem, S.; Bloem, K.A.; Carpenter, J.E.; Calkins, C.O. Inherited sterility in codling moth (Lepidoptera: Tortricidae): Effect of substerilizing doses of radiation on insect fecundity, fertility, and control. Ann. Entomol. Soc. Am. 1999, 92, 222–229. [Google Scholar] [CrossRef]
- Simmons, G.S.; Suckling, D.M.; Carpenter, J.E.; Addison, M.F.; Dyck, V.A.; Vreysen, M.J.B. Improved quality management to enhance the efficacy of the sterile insect technique for lepidopteran pests. J. Appl. Entomol. 2010, 134, 261–273. [Google Scholar] [CrossRef]
- Dyck, V.A. Rearing Codling Moth for the Sterile Insect Technique; FAO/IAEA Joint Division, Insect Control Section: Vienna, Austria, 2010; p. 195. [Google Scholar]
- Jones, V.P.; Doerr, M.; Brunner, J.F. Is bioix necessary for predicting codling moth (Lepidoptera: Tortricidae) emergence in Washington State apple orchards? J. Econ. Entomol. 2008, 101, 1651–1657. [Google Scholar] [CrossRef]
- VSNi Ltd. Genstat for Windows, 20; VSNi Ltd.: Hemel Hempstead, UK, 2020. [Google Scholar]
- Liebhold, A.M.; Berec, L.; Brockerhoff, E.G.; Epanchin-Niell, R.S.; Hastings, A.; Herms, D.A.; Kean, J.M.; McCullough, D.G.; Suckling, D.M.; Tobin, P.C.; et al. Eradication of invading insect populations: From concepts to applications. Annu. Rev. Entomol. 2016, 61, 335–352. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, V.J.; Manning, L.-A.; Cole, L.; Suckling, D.M.; El-Sayed, A.M. Efficacy of the pear ester as a monitoring tool for codling moth Cydia pomonella (Lepidoptera: Tortricidae) in New Zealand apple orchards. Pest Manag. Sci. 2008, 64, 209–214. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.M.; Cole, L.; Revell, J.; Manning, L.-A.; Twidle, A.; Knight, A.L.; Bus, V.G.; Suckling, D.M. Apple volatiles synergize the response of codling moth to pear ester. J. Chem. Ecol. 2013, 39, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Landolt, P.J.; Ohler, B.; Lo, P.; Cha, D.; Davis, T.S.; Suckling, D.M.; Brunner, J. N-Butyl sulfide as an attractant and coattractant for male and female codling moth (Lepidoptera: Tortricidae). Environ. Entomol. 2014, 43, 291–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Judd, G.; Gardiner, M. Towards eradication of codling moth in British Columbia by complimentary actions of mating disruption, tree banding and sterile insect technique: Five-year study in organic orchards. Crop. Prot. 2005, 24, 718–733. [Google Scholar] [CrossRef]
- Stringer, L.D.; Sullivan, N.J.; Sullivan, T.E.S.; Mitchell, V.J.; Manning, L.M.; Mas, F.; Hood-Nowotny, R.C.; Suckling, D.M. Attractiveness and competitiveness of irradiated light brown apple moths. Entomol. Exp. Appl. 2013, 148, 203–212. [Google Scholar] [CrossRef]
- Berec, L.; Angulo, E.; Courchamp, F. Multiple allee effects and population management. Trends Ecol. Evol. 2007, 22, 185–191. [Google Scholar] [CrossRef]
- Barclay, H.J. Models for pest control: Complementary effects of periodic releases of sterile pests and parasitoids. Theor. Popul. Biol. 1987, 32, 76–89. [Google Scholar] [CrossRef]
- Suckling, D.M.; Baker, G.; Salehi, L.; Woods, B. Is the combination of insecticide and mating disruption synergistic or additive in Lightbrown apple moth, epiphyas postvittana? PLoS ONE 2016, 11, e0160710. [Google Scholar] [CrossRef] [Green Version]
- Adams, C.G.; Schenker, J.H.; McGhee, P.S.; Gut, L.J.; Brunner, J.F.; Miller, J.R. Maximizing information yield from pheromone-baited monitoring traps: Estimating plume reach, trapping radius, and absolute density of Cydia pomonella (Lepidoptera: Tortricidae) in Michigan apple. J. Econ. Entomol. 2017, 110, 305–318. [Google Scholar] [CrossRef]
- Judd, G.J.; Gardiner, M.G.; Thistlewood, H.M. Seasonal variation in recapture of mass-reared sterile codling moth, Cydia pomonella (L.)(Lepidoptera: Tortricidae): Implications for control by sterile insect technique in British Columbia. J. Entomol. Soc. Br. Columbia 2004, 101, 29–44. [Google Scholar]
- Wood, T.G. Field observations on flight and oviposition of codling moth (Carpocapsa pomonella (L.)) and mortality of eggs and first-instar larvae in an integrated control orchard. N. Z. J. Agric. Res. 1965, 8, 1043–1059. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.T.; Tan, K.H. Alternative air vehicles for sterile insect technique aerial release. J. Appl. Entomol. 2013, 137, 126–141. [Google Scholar] [CrossRef]
- Bloem, K.A.; Bloem, S.; Carpenter, J.E. Impact of moth suppression/eradication programmes using the sterile insect technique or inherited sterility. In Sterile Insect Technique; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 677–700. [Google Scholar]
- Hofmeyr, J.H.; Carpenter, J.E.; Bloem, S.; Slabbert, J.P.; Hofmeyr, M.; Groenewald, S.S. Development of the sterile insect technique to suppress false codling moth Thaumatotibia leucotreta (Lepidoptera: Tortricidae) in Citrus Fruit: Research to Implementation (Part 1). Afr. Entomol. 2015, 23, 180–186. [Google Scholar] [CrossRef]
- Hofmeyr, J.H.; Groenewald, S.S.; Boersma, N. Development of the sterile insect technique to suppress Thaumatotibia leucotreta (lepidoptera: tortricidae) in citrus fruit: Commercialisation and expansion (Part 2). Afr. Entomol. 2019, 27, 289–299. [Google Scholar] [CrossRef]
- Barclay, H.J.; Matlock, R.; Gilchrist, S.; Suckling, D.M.; Reyes, J.; Enkerlin, W.R.; Vreysen, M.J.B. A conceptual model for assessing the minimum size area for an area-wide integrated pest management program. Int. J. Agron. 2011, 12, 409328. [Google Scholar] [CrossRef] [Green Version]









| Orchard Number | Number of Codling Moth Insecticides Applied | |||||||
|---|---|---|---|---|---|---|---|---|
| 2012–2013 | 2013–2014 | 2014–2015 | 2015–2016 | 2016–2017 | 2017–2018 | 2018–2019 | 2019–2020 | |
| A | 1 | 1–2 | 1 | 2 | 3–4 | 2 | 3 | 3 |
| B | 1 | 1 | 2 | 3–5 | 1–2 | 3 | 3 | 4 |
| C | 1 | 1–2 | 1–2 | 3–4 | 2–4 | 3 | 3 | 4 |
| D | 2 | 3–4 | 3–4 | 2–5 | 4–5 | 3 | 3 | 4 |
| E | 2 | 4 | 3 | 5 | 5–6 | 3 | 3 | 4 |
| F | 1 | 1–2 | 1–2 | 3–4 | 2–4 | 3 | 3 | 4 |
| G | NA | NA | NA | NA | NA | 6 | 4 | 3 |
| Orchard | 2014–2015 | 2015–2016 | 2016–2017 | 2017–2018 | 2018–2019 | 2019–2020 |
|---|---|---|---|---|---|---|
| Orchard A | 50 | 178 | 32 | 267 | 23 | 29 |
| Orchard B | 306 | 46 | 100 | 84 | 30 | 28 |
| Orchard C | 63 | 83 | 64 | 115 | 64 | 157 |
| Orchard D | 8 | 17 | 17 | 90 | ||
| Orchard E | 10 | 14 | 15 | 24 | ||
| Orchard F | 63 | 19 | 26 | 71 | ||
| Orchard G | 3 | 5 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horner, R.M.; Lo, P.L.; Rogers, D.J.; Walker, J.T.S.; Suckling, D.M. Combined Effects of Mating Disruption, Insecticides, and the Sterile Insect Technique on Cydia pomonella in New Zealand. Insects 2020, 11, 837. https://doi.org/10.3390/insects11120837
Horner RM, Lo PL, Rogers DJ, Walker JTS, Suckling DM. Combined Effects of Mating Disruption, Insecticides, and the Sterile Insect Technique on Cydia pomonella in New Zealand. Insects. 2020; 11(12):837. https://doi.org/10.3390/insects11120837
Chicago/Turabian StyleHorner, Rachael M., Peter L. Lo, David J. Rogers, James T. S. Walker, and David Maxwell Suckling. 2020. "Combined Effects of Mating Disruption, Insecticides, and the Sterile Insect Technique on Cydia pomonella in New Zealand" Insects 11, no. 12: 837. https://doi.org/10.3390/insects11120837