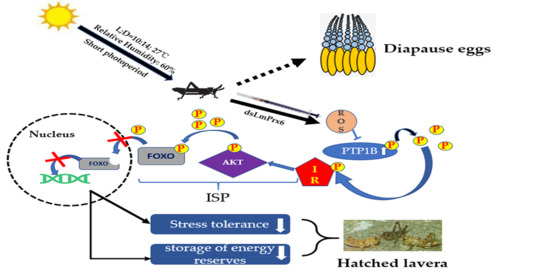
The Function of LmPrx6 in Diapause Regulation in Locusta migratoria Through the Insulin Signaling Pathway
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. cDNA Synthesis and LmPrx6 Cloning
2.3. Structure and Phylogenetic Analyses of LmPrx6
2.4. Synthesis and Injection of dsLmPrx6
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Diapause Rate Detection
2.7. LmPTP1B, LmIR, LmAkt, and LmFOXO Phosphorylation Level Detection
2.8. ROS Activity Detection
2.9. Catalase, Mn-SOD, Glycogen Synthase, and PEPCK Activities in the Diapause Eggs
3. Results
3.1. LmPrx6 Cloning and dsLmPrx6 Synthesis
3.2. Structure and Phylogenetic Analyses of LmPrx6
3.3. Functional Identification of LmPrx6 by RNAi
3.4. Impact of LmPrx6 on the Phosphorylation Level of Downstream PTP1B, IR, AKT, and FOXO
3.5. Impact of LmPrx6 on the mRNA Level of Genes Downstream of Foxo
3.6. ROS Activity Regulated by LmPrx6
3.7. Catalase, Mn-SOD, Glycogen synthase, and PEPCK activities Regulated by LmPrx6
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ISP | Insulin Signaling Pathway |
| Prxs | Peroxiredoxins |
| ROS | Reactive Oxygen Species |
| FOXO | forkhead box protein O |
| PTP1B | Protein-Tyrosine Phosphorylase 1B |
| AKT | RAC serine/threonine-protein kinase |
| IR | insulin receptor |
| Mn-SOD | Mn superoxide dismutase |
| PEPCK | phosphoenolpyruvate carboxy kinase |
| SP | Short photoperiod |
| LP | Long photoperiod |
| DR | Diapause rate |
| ELISA | Enzyme-Linked ImmunoSorbent Assay |
| RNAi | RNA interference |
References
- Masaki, S. Geographic Variation of Diapause in Insects; Bulletin of the Faculty of Agriculture Hirosaki University: Hirosaki, Japan, 1961; p. 7. Available online: http://hdl.handle.net/10129/432 (accessed on 12 May 2020).
- Denlinger, D.L. Regulation of diapause. Annu. Rev. Entomol. 2002, 47, 93–122. [Google Scholar] [CrossRef] [PubMed]
- MacRae, T.H. Gene expression, metabolic regulation and stress tolerance during diapause. Cell. Mol. Life Sci. 2010, 67, 2405–2424. [Google Scholar] [CrossRef] [PubMed]
- Hakomori, T.; Tanaka, S. Genetic control of diapause and other developmental traits in Japanese strains of the migratory locust, Locusta migratoria L.: Univoltine vs. bivoltine. Jpn. J. Syst. Entomol. 1992, 60, 319–328. [Google Scholar]
- Tanaka, S. The significance of embryonic diapause in a Japanese strain of the migratory locust, Locusta migratoria (Orthoptera: Acrididae). Jpn. J. Syst. Entomol. 1992, 60, 503–520. [Google Scholar]
- Tanaka, H. Embryonic diapause and life cycle in the migratory locust, Locusta migratoria L. (Orthoptera: Acrididae), in Kyoto. Appl. Entomol. Zool. 1994, 29, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Hao, K.; Ullah, H.; Aftab, R.J.; Nong, X.; Tu, X.; Zhang, Z. Functional identification of an FMRFamide-related peptide gene on diapause induction of the migratory locust, Locusta migratoria L. Genomics 2019, 112, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.O.; Cristina, S.R.; Ignacio, P.A.; Urbanek, P.; Steinbrenner, H.; Monsalve, M. Redox regulation of FoxO transcription factors. Redox Biol. 2015, 6, 51–72. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.Y.; Hench, J.; Ruvkun, G. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr. Biol. 2001, 11, 1950–1957. [Google Scholar] [CrossRef] [Green Version]
- Giannakou, M.E.; Goss, M.; Partridge, L. Role of dFOXO in lifespan extension by dietary restriction in Drosophila melanogaster: Not required, but its activity modulates the response. Aging Cell 2008, 7, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Libina, N.; Berman, J.R.; Kenyon, C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell 2003, 115, 489–502. [Google Scholar] [CrossRef] [Green Version]
- Warnhoff, K.; Murphy, J.T.; Kumar, S.; Schneider, D.L.; Peterson, M.; Hsu, S.; Guthrie, J.; Robertson, J.D.; Kornfeld, K. The DAF-16 FOXO transcription factor regulates natc-1 to modulate stress resistance in Caenorhabditis elegans, linking insulin/IGF-1 signaling to protein N-terminal acetylation. PLoS Genet. 2014, 10, e1004703. [Google Scholar] [CrossRef] [Green Version]
- Sim, C.; Denlinger, D.L. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc. Natl. Acad. Sci. USA 2008, 105, 6777–6781. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Choe, Y.G.; Kim, J.H.; Chang, K.T.; Lee, H.S.; Lee, D.S. Isoliquiritigenin impairs insulin signaling and adipocyte differentiation through the inhibition of protein-tyrosine phosphatase 1B oxidation in 3T3-L1 preadipocytes. Food Chem. Toxicol. 2016, 93, 5–12. [Google Scholar] [CrossRef]
- Tiganis, T. Reactive oxygen species and insulin resistance: The good, the bad and the ugly. Trends Pharmacol. Sci. 2011, 32, 82–89. [Google Scholar] [CrossRef]
- Bashan, N.; Kovsan, J.; Kachko, I.; Ovadia, H.; Rundich, A. Positive and Negative Regulation of Insulin Signaling by Reactive Oxygen and Nitrogen Species. Physiol. Rev. 2009, 89, 27–71. [Google Scholar] [CrossRef] [Green Version]
- Mahadev, K.; Wu, X.; Zilbering, A.; Zhe, L.; Lawrence, J.T.R.; Goldstein, B.J. Hydrogen Peroxide Generated during Cellular Insulin Stimulation Is Integral to Activation of the Distal Insulin Signaling Cascade in 3T3-L1 Adipocytes. J. Biol. Chem. 2001, 276, 48662–48669. [Google Scholar] [CrossRef] [Green Version]
- Brunet, A.; Bonni, A.; Zigmand, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef] [Green Version]
- Denlinger, D.L.; Armbruster, P.A. Mosquito diapause. Annu. Rev. Entomol. 2014, 59, 73–93. [Google Scholar] [CrossRef]
- Chen, Y.R.; Jiang, T.; Zhu, J.; Xie, Y.C.; Tan, Z.C.; Chen, Y.H.; Tang, S.M.; Hao, B.F.; Wang, S.P.; Huang, J.S.; et al. Transcriptome sequencing reveals potential mechanisms of diapause preparation in bivoltine silkworm Bombyx mori (Lepidoptera: Bombycidae). Comp. Biochem. Physiol. Part D Genom. Proteom. 2017, 24, 68–78. [Google Scholar] [CrossRef]
- Wardhaugh, K.G. The effects of temperature and moisture on the inception of diapause in eggs of the Australian plague locust, Chortoicetes terminifera Walker (Orthoptera: Acrididae). Aust. J. Ecol. 2010, 5, 187–191. [Google Scholar] [CrossRef]
- Hao, K.; Jarwar, A.R.; Ullah, H.; Tu, X.; Nong, X.; Zhang, Z. Transcriptome Sequencing Reveals Potential Mechanisms of the Maternal Effect on Egg Diapause Induction of Locusta migratoria. Int. J. Mol. Sci. 2019, 20, 1974. [Google Scholar] [CrossRef] [Green Version]
- Ezoysa, M.; Ryu, J.H.; Chung, H.C.; Kim, C.H.; Nikapitiya, C.; Oh, C.; Kim, H.; Revathy, K.S.; Whang, I.; Lee, J. Molecular characterization, immune responses and DNA protection activity of rock bream (Oplegnathus fasciatus), peroxiredoxin 6 (Prx6). Fish Shellfish Immunol. 2012, 33, 28–35. [Google Scholar] [CrossRef]
- Jin, Y.Z.; Sun, H.N.; Liu, Y.; Lee, D.H.; Kim, J.S.; Kim, S.K.; Jiao, B.Y.; Han, Y.H.; Jin, M.H.; Shen, G.N.; et al. Peroxiredoxin V Inhibits Emodin-induced Gastric Cancer Cell Apoptosis via the ROS/Bcl2 Pathway. In Vivo 2019, 33, 1183–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, J.; Ikeda, Y. Advances in our understanding of peroxiredoxin, a multifunctional, mammalian redox protein. Redox Rep. 2002, 7, 123–130. [Google Scholar] [CrossRef]
- Hall, A.; Nelson, K.; Poole, L.B.; Karplus, P.A. Structure-based Insights into the Catalytic Power and Conformational Dexterity of Peroxiredoxins. Antioxid. Redox Signal. 2011, 15, 795–815. [Google Scholar] [CrossRef] [Green Version]
- Nelson, K.J.; Knutson, S.T.; Soito, L.; Klomsiri, C.; Poole, L.B.; Fetrow, J.S. Analysis of the peroxiredoxin family: Using active-site structure and sequence information for global classification and residue analysis. Proteins-Struct. Funct. Bioinform. 2011, 79, 947–964. [Google Scholar] [CrossRef] [Green Version]
- Trivelli, X.; Krimm, I.; Ebel, C.; Verdoucq, L.; Valerie, P.M.; Chartier, Y.; Tsan, P.; Lauquin, G.; Meyer, Y.; Lancelin, J.M. Characterization of the yeast peroxiredoxin Ahp1 in its reduced active and overoxidized inactive forms using NMR. Biochemistry 2003, 42, 14139–14149. [Google Scholar] [CrossRef]
- Rhee, S.G.; Yang, K.S.; Kang, S.W.; Woo, H.A.; Chang, T.S. Controlled elimination of intracellular H(2)O(2): Regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxid. Redox Signal. 2005, 7, 619. [Google Scholar] [CrossRef]
- Woo, H.A.; Yim, S.H.; Shin, D.H.; Yu, D.Y.; Rhee, S.G. Inactivation of Peroxiredoxin I by Phosphorylation Allows Localized H2O2 Accumulation for Cell Signaling. Cell 2010, 140, 517–528. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.J.; Kang, S.W.; Yang, C.H.; Rhee, S.G.; Ryu, S.E. Crystal structure of a novel human peroxidase enzyme at 2.0 A resolution. Nat. Struct. Biol. 1998, 5, 400–406. [Google Scholar] [CrossRef]
- Deponte, M.; Becker, K. Biochemical characterization of Toxoplasma gondii 1-Cys peroxiredoxin 2 with mechanistic similarities to typical 2-Cys Prx. Mol. Biochem. Parasitol. 2005, 140, 87–96. [Google Scholar] [CrossRef]
- Singh, R.; Karakoti, A.S.; Self, W.; Seal, S.; Singh, S. Redox-Sensitive Cerium Oxide Nanoparticles Protect Human Keratinocytes from Oxidative Stress Induced by Glutathione Depletion. Langmuir 2016, 32, 12202–12211. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Marcocci, L.; Shimomura, T.; Tatenaka, Y.; Ohuchi, Y.; Brelidze, T.I. Protein Redox State Monitoring Studies of Thiol Reactivity. Antioxidants 2019, 8, 143. [Google Scholar] [CrossRef] [Green Version]
- Helm, R.R.; Martín-Díaz, M.L.; Tarrant, A.M. Phylogenetic analysis of cnidarian peroxiredoxins and stress-responsive expression in the estuarine sea anemone Nematostella vectensis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2018, 221, 32–43. [Google Scholar] [CrossRef]
- Tu, D.D.; Zhou, Y.L.; Gu, W.B.; Zhu, Q.H.; Xu, B.P.; Zhou, Z.K.; Liu, Z.P.; Wang, C.; Chen, Y.Y.; Shu, M.A. Identification and characterization of six peroxiredoxin transcripts from mud crab Scylla paramamosain: The first evidence of peroxiredoxin gene family in crustacean and their expression profiles under biotic and abiotic stresses. Mol. Immunol. 2018, 93, 223–235. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, X.; Zheng, L.; Li, Z.; Zhao, X.; Lai, W.; Shen, H.; Lv, J.; Yang, G.; Wang, Q.; et al. Peroxiredoxin 6 Is a Crucial Factor in the Initial Step of Mitochondrial Clearance and Is Upstream of the PINK1-Parkin Pathway. Antioxid. Redox Signal. 2016, 24, 486–501. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Zuckerkandl, E.; Pauling, L. Evolutionary divergence and convergence in proteins. In Evolving Genes and Proteins; Bryson, V., Vogel, H.J., Eds.; Academic Press: New York, NY, USA, 1965; pp. 97–166. [Google Scholar]
- Hao, K.; Wang, J.; Tu, X.B.; Douglas, W.W.; Zhang, Z.H. Transcriptomic and proteomic analysis of Locusta migratoria eggs at different embryonic stages: Comparison for diapause and nondiapause regimes. J. Integr. Agric. 2017, 16, 60345–60347. [Google Scholar] [CrossRef] [Green Version]
- Sim, C.; Denlinger, D.L. Catalase and superoxide dismutase-2 enhance survival and protect ovaries during overwintering diapause in the mosquito Culex pipiens. J. Insect Physiol. 2011, 57, 628–634. [Google Scholar] [CrossRef] [Green Version]
- Hahn, D.A.; Denlinger, D.L. Energetics of insect diapause. Annu. Rev. Entomol. 2011, 56, 103–121. [Google Scholar] [CrossRef]
- Rhee, S.G.; Woo, H.A. Multiple functions of peroxiredoxins: Peroxidases, sensors and regulators of the intracellular messenger H2O2, and protein chaperones. Antioxid Redox Signal. 2011, 15, 781–794. [Google Scholar] [CrossRef]
- Zhang, X.S.; Wang, T.; Lin, X.W.; Denlinger, D.L.; Xu, W.H. Reactive oxygen species extend insect life span using components of the insulin-signaling pathway. Proc. Natl. Acad. Sci. USA. 2017, 114, E7832–E7840. [Google Scholar] [CrossRef] [Green Version]
- Sugahara, R.; Tanaka, S.; Jouraku, A.; Shiotsuki, T. Geographic variation in RNAi sensitivity in the migratory locust. Gene 2017, 605, 5–11. [Google Scholar] [CrossRef]
- Martin, G.M.; Wang, L.M.; Sun, X.J.; Zhang, Y.T.; Lynne, Y.; Schlessinger, J.; Pierce, J.H.; White, M.F. Role of IRS-1-GRB-2 complexes in insulin signaling. Mol. Cell. Biol. 1994, 14, 3577–3587. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, A.; Dutta, A.; Dandapat, J.; Samanta, L. Low H2O2 and enhanced oxidative resistance in the diapause-destined pupa of silkworm, Antheraea mylitta (Lepidoptera: Saturniidae) suggest their possible involvement in dormancy and lifespan extension. BMC Zool. 2018, 3, 1. [Google Scholar] [CrossRef]
- Almeida, M. Unraveling the role of FoxOs in bone—Insights from mouse models. Bone 2011, 49, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Chiu, J.F.; Mossman, B.T.; Fukagawa, N.K. Down-regulation of manganese-superoxide dismutase through phosphorylation of FOXO3a by Akt in explanted vascular smooth muscle cells from old rats. J. Biol. Chem. 2006, 281, 40429–40439. [Google Scholar] [CrossRef] [Green Version]
- Malik, A.I.; Storey, K.B. Transcriptional regulation of antioxidant enzymes by FoxO1 under dehydration stress. Gene 2011, 485, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Hao, K.; Tu, X.; Ullah, H.; McNeill, M.R.; Zhang, Z. Novel Lom-dh genes play potential role in promoting egg diapause of Locusta migratoria L. Front. Physiol. 2019, 10, 767. [Google Scholar] [CrossRef]
- Lin, J.L.; Lin, P.L.; Gu, S.H. Phosphorylation of glycogen synthase kinase-3β in relation to diapause processing in the silkworm, Bombyx mori. J. Insect Physiol. 2009, 55, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Nimmo, H.G.; Proud, C.G. How does insulin stimulate glycogen synthesis? Biochem. Soc. Symp. 1978, 43, 69–95. [Google Scholar]
- Cross, D.A.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995, 378, 785. [Google Scholar] [CrossRef]
- Embi, N.; Rylatt, D.B.; Cohen, P. Glycogen Synthase Kinase-3 from Rabbit Skeletal Muscle: Separation from Cyclic-AMP-Dependent Protein Kinase and Phosphorylase Kinase. Eur. J. Biochem. 1980, 107, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; An, T.; Huang, F.; Wang, M.; Liu, C.; Mao, J.; Zhang, L. Comparative transcriptome and iTRAQ proteome analyses reveal the mechanisms of diapause in Aphidius gifuensis Ashmead (Hymenoptera: Aphidiidae). Front. Physiol. 2018, 9, 1697. [Google Scholar] [CrossRef]
- Ragland, G.J.; Denlinger, D.L.; Hahn, D.A. Mechanisms of suspended animation are revealed by transcript profiling of diapause in the flesh fly. Proc. Natl. Acad. Sci. USA 2010, 107, 14909–14914. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Geng, S.L.; Guan, Y.M.; Xu, W.H. Deacetylation of metabolic enzymes by Sirt2 modulates pyruvate homeostasis to extend insect lifespan. Aging 2018, 10, 1053. [Google Scholar] [CrossRef]







| Primers | Primer Sequences (5′–3′) | Purpose |
|---|---|---|
| LmPrx6-1F LmPrx6-1R | ACGTAGTTGTTGCCGGAG | Clone of the target gene |
| CGCTACAGAGAGAAGTCTACA | ||
| LmPrx6-2F LmPrx6-2R | TAATACGACTCACTATAGGACGTAGTTGTTGCCGGAG | Synthesis of the dsRNA |
| TAATACGACTCACTATAGGCGCTACAGAGAGAAGTCTACA | ||
| LmPrx6-3F | TGGAAAGGAAACTCGTGG | RT-qPCR |
| LmPrx6-3R | TTGTCACAGGAGAGAGCCA | |
| foxo-F | AGAACTCGATCCGGCACAAC | |
| foxo-R | CGCCTCCACCTTCTTCTTGA | |
| Catalase-F | GGTATTTGGGATTTGGTGG | |
| Catalase-R | GGGTTGTCTCTGGTCTAAGTG | |
| Glycogen synthase-F | AAAGTTCCTCGCTCTCCACG | |
| Glycogen synthase-R | ACATCAGCACCCTTGTTTCC | |
| Mn-SOD-F | CAGACCAACGCTACTCTCGC | |
| Mn-SOD-R | TAATGACCTCCCAAATGGCG | |
| Phosphoenolpyruvate-F | ATTTAGAAACGGGAGGCAAG | |
| Phosphoenolpyruvate-R | AGGTGGATTACTCGGATGAC | |
| βactin-F | GTTACAAACTGGGACGACAT | qPCR reference gene |
| βactin-R | AGAAAGCACAGCCTGAATAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Cui, D.-N.; Ullah, H.; Li, S.; Pan, F.; Xu, C.-M.; Tu, X.-B.; Zhang, Z.-H. The Function of LmPrx6 in Diapause Regulation in Locusta migratoria Through the Insulin Signaling Pathway. Insects 2020, 11, 763. https://doi.org/10.3390/insects11110763
Chen J, Cui D-N, Ullah H, Li S, Pan F, Xu C-M, Tu X-B, Zhang Z-H. The Function of LmPrx6 in Diapause Regulation in Locusta migratoria Through the Insulin Signaling Pathway. Insects. 2020; 11(11):763. https://doi.org/10.3390/insects11110763
Chicago/Turabian StyleChen, Jun, Dong-Nan Cui, Hidayat Ullah, Shuang Li, Fan Pan, Chao-Min Xu, Xiong-Bing Tu, and Ze-Hua Zhang. 2020. "The Function of LmPrx6 in Diapause Regulation in Locusta migratoria Through the Insulin Signaling Pathway" Insects 11, no. 11: 763. https://doi.org/10.3390/insects11110763