Commercially Available Essential Oil Formulas as Repellents Against the Stored-Product Pest Alphitobius diaperinus
Abstract
:1. Introduction
2. Materials and Methods
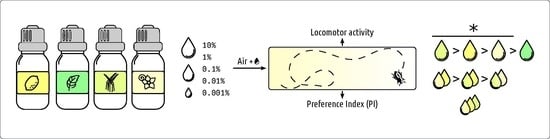
2.1. Essential Oils
2.2. Insects
2.3. Behavioural Test
2.4. Statistical Analysis
3. Results
3.1. Behavioural Effects—Single Oils
3.1.1. Mint EO
3.1.2. Lemon EO
3.1.3. Citronella EO
3.1.4. Vanilla EO
3.2. Behavioural Effects—Oil Mixtures
3.2.1. Citronella/Vanilla EOs
3.2.2. Lemon/Vanilla EOs
3.2.3. Citronella/Lemon EOs
3.2.4. Citronella/Vanilla/Lemon EOs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rumbos, C.I.; Karapanagiotidis, I.T.; Mente, E.; Athanassiou, C.G. The lesser mealworm Alphitobius diaperinus: A noxious pest or a promising nutrient source? Rev. Aquac. 2018. [Google Scholar] [CrossRef]
- Goodwin, M.; Waltman, W. Transmission of Eimeria, viruses, and bacteria to chicks: Darkling beetles (Alphitobius diaperinus) as vectors of pathogens. J. Appl. Poult. 1996, 5. [Google Scholar] [CrossRef]
- Tavassoli, M.; Allymehr, M.; Oftad, H. Prevelance of coleopteran species in the litter of commercialy reabirdsred. Iran. J. Vet. Med. 2011, 5, 232–238. [Google Scholar]
- Despins, J.L.; Turner, J.E.C.; Pfeiffer, D.G. Evaluation of methods to protect poultry house insulation from infestations by lesser mealworm (Coleoptera: Tenebrionidae). J. Agric. Entomol. 1991, 8, 209–217. [Google Scholar]
- Hamm, R.L.; Kaufman, P.E.; Reasor, C.A.; Rutz, D.A.; Scott, J.G. Resistance to cyfluthrin and tetrachlorvinphos in the lesser mealworm, Alphitobius diaperinus, collected from the eastern United States. Pest Manag. Sci. 2006, 62, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Lambkin, T.; Rice, S. Baseline responses of Alphitobius diaperinus (Coleoptera: Tenebrionidae) to cyfluthrin and detection of strong resistance in field populations in eastern Australia. J. Econ. Entomol. 2006, 99, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Boada, L.D.; Sangil, M.; Alvarez-León, E.E.; Hernández-Rodríguez, G.; Henríquez-Hernández, L.A.; Camacho, M.; Zumbado, M.; Serra-Majem, L.; Luzardo, O.P. Consumption of foods of animal origin as determinant of contamination by organochlorine pesticides and polychlorobiphenyls: Results from a population-based study in Spain. Chemosphere 2014, 114, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Misra, G.; Pavlostathis, S. Biodegradation kinetics of monoterpenes in liquid and soil-slurry systems. Appl. Microbiol. Biotechnol. 1997, 5, 572–577. [Google Scholar] [CrossRef]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Benelli, G.; Flamini, G.; Canale, A.; Molfetta, I. Repellence of Hyptis suaveolens whole essential oil and major constituents against adults of the granary weevil Sitophilus granarius. Bull. Insect. 2012, 65, 177–183. [Google Scholar]
- Szołyga, B.; Gniłka, R.; Szczepanik, M.; Szumny, A. Chemical composition and insecticidal activity of Thuja occidentalis and Tanacetum vulgare essential oils against larvae of the lesser mealworm, Alphitobius diaperinus. Entomol. Exp. Appl. 2014, 151, 1–10. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The Use of Push-Pull Strategies in Integrated Pest Management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef] [PubMed]
- Baran, B.; Krzyżowski, M.; Cup, M.; Janiec, J.; Grabowski, M.; Francikowski, J. Repellent effect of volatile fatty acids on lesser mealworm (Alphitobius diaperinus). Insects 2018, 9, 35. [Google Scholar] [CrossRef]
- Becker, H.J. The genetics of chemotaxis in Drosophila melanogaster: Selection for repellent insensitivity. MGG Mol. Gen. Genet. 1970, 107, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Krause Pham, C.; Ray, A. Conservation of Olfactory Avoidance in Drosophila Species and Identification of Repellents for Drosophila suzukii. Sci. Rep. 2015, 5, 11527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garud, A.; Ganesan, K.; Prakash, S.; Vijayaraghavan, R.; Shinde, C.K. Behavioral responses and bioefficacy of some aromatic amides against Aedes aegypti. J. Econ. Entomol. 2011, 104, 1369–1378. [Google Scholar] [CrossRef]
- Mohan, S.; Fields, P. A simple technique to assess compounds that are repellent or attractive to stored-product insects. J. Stored Prod. Res. 2002, 38, 23–31. [Google Scholar] [CrossRef]
- Bougherra, H.H.; Bedini, S.; Flamini, G.; Cosci, F.; Belhamel, K.; Conti, B. Pistacia lentiscus essential oil has repellent effect against three major insect pests of pasta. Ind. Crops Prod. 2015, 63, 249–255. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Girardi, J.; Cosci, F.; Conti, B. Not just for beer: Evaluation of spent hops (Humulus lupulus L.) as a source of eco-friendly repellents for insect pests of stored foods. J. Pest Sci. 2015, 88, 583–592. [Google Scholar] [CrossRef]
- Burkholder, W.; Barak, A. Suffocation-type insect trap with pitfall and attractant. U.S. Patent 4,581,845, 1986. [Google Scholar]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine Int. J. Phyther. Phytopharm. 2010, 17, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Tak, J.-H.; Isman, M.B. Enhanced cuticular penetration as the mechanism for synergy of insecticidal constituents of rosemary essential oil in Trichoplusia ni. Sci. Rep. 2015, 5, 12690. [Google Scholar] [CrossRef] [Green Version]
- Amer, A.; Mehlhorn, H. Repellency effect of forty-one essential oils against Aedes, Anopheles, and Culex mosquitoes. Parasitol. Res. 2006, 99, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Regnault-Roger, C.; Vincent, C.; Arnason, J. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2011, 57, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef]
- Soujanya, P.L.; Sekhar, J.C.; Kumar, P.; Sunil, N.; Prasad, C.V.; Mallavadhani, U.V. Potentiality of botanical agents for the management of post harvest insects of maize: A review. J. Food Sci. Technol. 2016, 53, 2169–2184. [Google Scholar] [CrossRef] [PubMed]
- Erler, F.; Ulug, I.; Yalcinkaya, B. Repellent activity of five essential oils against Culex pipiens. Fitoterapia 2006, 77, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Shen, L.; Yang, J. Fumigant, contact, and repellent activities of essential oils against the darkling beetle, Alphitobius diaperinus. J Insect Sci. 2014, 14. [Google Scholar] [CrossRef]
- Jeyasankar, A.; Chennaiyan, V.; Chinnamani, T. Evaluation of Five Essential Plant Oils as a Source of Repellent and Larvicidal Activities Against Larvae of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Entomol. 2016, 13, 98–103. [Google Scholar] [CrossRef]
- Chan, H.K.; Hersperger, F.; Marachlian, E.; Smith, B.H.; Locatelli, F.; Szyszka, P.; Nowotny, T. Odorant mixtures elicit less variable and faster responses than pure odorants. PLOS Comput. Biol. 2018, 14, e1006536. [Google Scholar] [CrossRef] [PubMed]
- Dobetsberger, C.; Buchbauer, G. Actions of essential oils on the central nervous system: An updated review. Flavour Fragr. J. 2011, 26, 300–316. [Google Scholar] [CrossRef]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular Targets for Components of Essential Oils in the Insect Nervous System—A Review. Molecules 2017, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Osborne, R.H. Insect neurotransmission: neurotransmitters and their receptors. Pharmacol. Ther. 1996, 69, 117–142. [Google Scholar] [CrossRef]
- Enan, E.E. Molecular and pharmacological analysis of an octopamine receptor from american cockroach and fruit fly in response to plant essential oils. Arch. Insect Biochem. Physiol. 2005, 59, 161–171. [Google Scholar] [CrossRef]
- Ceccarelli, I.; Lariviere, W.R.; Fiorenzani, P.; Sacerdote, P.; Aloisi, A.M. Effects of long-term exposure of lemon essential oil odor on behavioral, hormonal and neuronal parameters in male and female rats. Brain Res. 2004, 1001, 78–86. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francikowski, J.; Baran, B.; Cup, M.; Janiec, J.; Krzyżowski, M. Commercially Available Essential Oil Formulas as Repellents Against the Stored-Product Pest Alphitobius diaperinus. Insects 2019, 10, 96. https://doi.org/10.3390/insects10040096
Francikowski J, Baran B, Cup M, Janiec J, Krzyżowski M. Commercially Available Essential Oil Formulas as Repellents Against the Stored-Product Pest Alphitobius diaperinus. Insects. 2019; 10(4):96. https://doi.org/10.3390/insects10040096
Chicago/Turabian StyleFrancikowski, Jacek, Bartosz Baran, Mikołaj Cup, Jakub Janiec, and Michał Krzyżowski. 2019. "Commercially Available Essential Oil Formulas as Repellents Against the Stored-Product Pest Alphitobius diaperinus" Insects 10, no. 4: 96. https://doi.org/10.3390/insects10040096