A Comprehensive Review of Water-Based Nanolubricants
Abstract
:1. Introduction
2. Preparation of Water-Based Nanolubricants
2.1. Preparation Methods
2.2. Nanoadditives in Water
2.2.1. Pure Metals
2.2.2. Metal and Non-Metal Oxides
2.2.3. Metal Sulphides
2.2.4. Carbon-Based Materials
2.2.5. Composites
2.2.6. Nitrides
2.2.7. Carbides
2.2.8. Others
| Types | Nanoadditives | Size | Shape | Concentration | Stirring | Stability Duration | References |
|---|---|---|---|---|---|---|---|
| Metals | Cu | 3 nm | Spherical | 0.1–2.0 wt.% | Magnetic stirring for 10, 20 min | - | Zhao et al. [46] |
| 2 nm | - | 0.5–5 wt.% | - | Zhang et al. [47] | |||
| Ag | 10–100 nm | - | 1 g/L | - | 5 to 19 days | Odzak et al. [97] | |
| Au | 26.7 nm | - | 0.018 vol% | Irradiation for 1–18 h | 1 month | Kim et al. [98] | |
| Metal and non-metal oxides | Al2O3 | 0.197 µm | Spherical | - | Ultrasonication for 10 min | Few minutes | Radice et al. [48] |
| 30, 150, and 500 nm | Spherical | 0.2–8 wt.% | Ultrasonication for 10 min | 3 days | He et al. [16] | ||
| CeO2 | 10–40 nm | - | 0.05 wt.% | Ultrasonication for 2 min | 4 days | Zhao et al. [53] | |
| CuO | 60 nm wide and 230 nm long | Nanorod or spindle | 0.1–2.0 wt.% | Magnetic stirring for 60 min | 8 h | Zhao et al. [54] | |
| γ-Fe2O3 | 5 nm | - | 0.1–1 wt.% | - | 1 h | Pardue et al. [55] | |
| Fe3O4 | - | Chain like | 1 wt.% | Ultrasonication for 1 h | 40 days | Lv et al. [99] | |
| MoO3 | 80–100 nm | 2D | 0.1, 0.2, 0.3, 0.4, 0.5 wt.% | - | - | Sun et al. [56] | |
| SiO2 | 100 nm | - | 0.5 wt.% | - | 1 h | Ding et al. [58] | |
| 30 nm | Spherical | 0.1–1 wt.% | Magnetic stirring for 10 min | - | Bao et al. [59,100] | ||
| 20 nm | Spherical | 0.5 wt.% | - | 30 days | Lv et al. [101] | ||
| TiO2 | 30 nm | - | 0.1–2.0 wt.% | Stirring for 0.5 h | - | Gao et al. [102] | |
| 20 nm | Spherical | 0.1, 0.2, 0.4, 0.8, 1.6 wt.% | Mechanical stirring and ultrasonic | - | Gu et al. [103] | ||
| 0.2–0.4 µm | - | 0.25, 0.5, 1.0 wt.NaPa/TiO2 % | Stirring and ultrasonication | - | Ohenoja et al. [104] | ||
| 40 nm | Non-spherical | 1.5 vol.% | Ultrasonication for 2 h | - | Najiha et al. [105] | ||
| 15 nm | Spherical | 0.1, 0.5, 1.0, 1.5, 2.0 vol.% | Ultrasonic vibration | Several days | Kayhani et al. [106] | ||
| 20 nm | Spherical | 0.2–8.0 wt.% | Mechanical and ultrasonication for 10 min | 7 days | Wu et al. [13,14,15,27,29] | ||
| 20 nm | Spherical | 0.5–4.0 wt.% | Ultrasonic stirring | - | Huo et al. [26] | ||
| 300 nm | Spherical | 2.0–4.0 wt.% | Mechanical stirring | 48 h | Wu et al. [24] | ||
| 50 nm | Spherical | 0, 0.25, 0.5, 0.75, 1.0 wt.% | Ultrasonication for 30 min | - | Kong et al. [49] | ||
| ~20 nm | - | 0.03, 0.05, 0.07 wt.% | Magnetic stirring | - | Ukamanal et al. [107] | ||
| 90 nm | - | 0.5–1.5 wt.% | Magnetic stirring | 7 days | Meng et al. [52] | ||
| 20 to 50 nm | - | 0.1, 0.4, 0.7, 1.0, 2.0, 3.0, 4.0, 5.0 wt.% | - | - | Sun et al. [50] | ||
| 40 nm | - | 0.1, 0.5, 1, 2, 4 wt.% | Ultrasonication | 30 min | Wang et al. [108] | ||
| 20–25 nm | - | - | Magnetic heat for 1 h | 7 days | Zhu et al. [51] | ||
| SiO2, TiO2, ZnO | 100 nm | Spherical | ASNPs 3 wt.%, AZNPs 1 wt.%, ATNPs 1 wt.% | Magnetic stirring for 6 h | - | Cui et al. [109] | |
| ZnO, CuO | ZnO (4.5 & 27 nm); CuO (7.5, 45 nm) | - | 1 g/L | Ultrasonication for 30 min | 19 days | Odzak et al. [97] | |
| WO3 | 50 nm | Spherical | 0–1 wt.% | Magnetic stirring for 2 h | 5 days | Xiong et al. [57] | |
| Metal sulphides | Ag2S | 2–10 nm | - | - | Sonication for 10 min | 1 h | Kuznetsova [61] |
| CuS | 4 nm | Uniform spherical | 0.1 to 2.0 wt.% | Magnetic stirring for 10 min, 1 h | 2 days | Zhao et al. [62] | |
| MoS2 | - | - | 0.1 g | - | 10 days | Wu et al. [110] | |
| 100–300 nm | layered | - | Stirring for 10, 20 min | - | Zhang et al. [111] | ||
| height 3.5 nm | Chain like layered | 0.05 and 0.1 wt.% | Ultrasonication for 1, 2, 3 h | 10 days | Wang et al. [64] | ||
| 100 nm | - | 0.3–0.5 wt.% | Magnetic stirring for 10 min | 16 h | Meng et al. [63] | ||
| Composites | graphene-SiO2 | Graphene (5 nm thick, interlayer distance 0.34 nm); SiO2 (30 nm) | SiO2 spherical, graphene multi-layered sheet | Graphene:SiO2 (0.4:0.1, 0.3:0.2, 0.2:0.3, and 0.1:0.4) | Stirring for 1hr, ultrasonic bathing for 2 h | - | Xie et al. [112] |
| GO-SiO2 | GO (1−2 nm thick); SiO2 (30−40 nm) | GO sheet wrinkled folded | 0.03–0.5 wt.% | Magnetic stirring for 24 h | 60 days | Guo et al. [83] | |
| GO-SiO2 | GO (4–6 nm); SiO2 (25–30 nm) | GO lamellar wrinkled, SiO2 spherical | 0.04, 0.08, 0.12, 0.16 and 0.20 wt.%. | Mechanical stirring for 30 min, ultrasonication | - | Huang et al. [19] | |
| CNT-SiO2 | CNT (inner diameter 8 nm, outer diameter 15 nm); SiO2 (30 nm) | SiO2 spherical, CNT tubular | 0.5 wt.% | Magnetic stirring for 1 h, ultrasonic bathing for 2 h | - | Xie et al. [113] | |
| GO-TiO2 | TiO2 (25 nm) | TiO2 spherical | 0.5 wt.% (0.3 wt.% GO-0.2 wt.% TiO2) | Stirring for 20 min, sonicating for 40 min | 30 days | Du et al. [84] | |
| GO-Al2O3 | GO (4~6 nm thick, 10~50 𝜇m lateral sizes), Al2O3 (15, 30 & 135 nm) | Layered | 0.25, 0.5, 1.0 and 2.0 wt.% | Magnetic stirring for 30 min, ultrasonic probe for 30 min | 1 h | Huang et al. [20] | |
| GO-Al2O3 | GO (10–50 μm in diameter; 1–2 nm thick); Al2O3 (30 nm) | GO layered; Al2O3 near-spherical | 0.04, 0.08, 0.12, 0.16, and 0.20 wt.% | Mechanical stirring for 10 min, ultrasonic agitation process | - | Huang et al. [21] | |
| GO-TiO2/ZrO2 | TiO2/ZrO2 (25 nm); GO (3–5 nm thick, 1.5–5.5 μm lateral) | 2D GO; zero dimension TiO2/ZrO2 | 0.5 wt.% | Magnetic stirring and ultrasonication for—30 min, 1 h | - | Huang et al. [12] | |
| GO-TiO2-Ag | - | - | 0.05 wt.% | Sonication for 4 h | - | Zayan et al. [114] | |
| PTEE-SiO2 | 413.6 nm (SiO2 layer 20–30 nm) | PTFE rod-like or spherical | 0.2, 1 and 3 wt.% (PTFE:SiO2–0.57:0.43) | Ultrasonication for 20 min | 12 h | Wang et al. [115] | |
| Cu-SiO2 | 20 nm average (Silica layer thick 2 nm) | network-like silica, Cu spherical | 0, 0.5, 1.0, 1.5, 2.0 wt.% | Magnetic stirring | - | Zhang et al. [116] | |
| - | Sphere | 0.4 wt.% | Magnetic stirring for 15 min | 30 days | Liu et al. [117] | ||
| MoS2-Al2O3 | MoS2-Al2O3 (144.8 nm), MoS2 (178.6 nm), Al2O3 (35.4 nm) | Laminar | 2.0 wt.% | Electro-magnetic stirring | 168 h | He et al. [118] | |
| Al2O3, MoS2, hBN, and WS2 | Al2O3 (<100 nm), hBN (70−80 nm), MoS2 (80−100 nm), WS2 (80−100 nm) | Al2O3 (spherical); hBN, MoS2, and WS2 (layered structure) | 1% each | Ultrasonic bath for 1 h | 24 h | Kumar et al. [119] | |
| Fe3O4-MoS2 | MoS2 (100–400 nm), Fe3O4 (10 nm), Fe3O4 on MoS2 (30–60 nm) | Laminated structure | 0.3, 0.6, 0.9, 1.2 wt.% | Ultrasonication | - | Zheng et al. [86] | |
| MWCNT-Fe2O3 | Fe2O3 (20–30 nm); MWCNT (10–30 µm length, 10–20 nm outer diameter, 3–5 nm inner diameter) | Multi-walled carbon nanotube | 0.1–1.5 vol.% (Fe2O3 80%, MWCNT 20%) | Sonication for 120 min | 1 month | Giwa et al. [120] | |
| Ag-C | 350–400 nm (C shell 100–120 nm thick) Ag 130–180 nm | Core spherical, NPs elliptical (core like short rod) | Ag 28 wt.% in Ag-C | Magnetic stirring for 30 min, ultrasonication for 60 min | 5 days | Song et al. [121] | |
| TiO2-Ag | TiO2 (40 nm) | Ellipsoidal | 0.05, 0.1, 0.1, 0.25, 0.3 wt.% | Magnetic stirring for 2 h | 1 month | Li et al. [85] | |
| ZnO-Al2O3 | ZnO (70 nm), Al2O3 (45 nm) | ZnO elongated, Al2O3 spherical | 0.1–23 wt.% | Ultrasonic bath for 30 min | - | Gara et al. [122] | |
| WO3-Mn3B7O13Cl | 22.4 nm | Spherical | 0.0, 0.1, 0.3, 0.5, 0.7and 0.9 wt.% | Ultrasonic vibration for 1 h | 48 h | Liang et al. [123] | |
| Carbon-based materials | Carbon | outer diameter ~177 nm | Toroidal | 2.0, 1.5, 1.2, 1.0, 0.5, and 0.1 wt.% | Magnetic stirring for 60 h | 4 months | Peña-Parás et al. [65] |
| 130, 170, 200 and 250 nm | Spherical | 0.05, 0.1, 0.15, 0.2, 0.3 wt.% | Ultrasonication | 5–10 h | Wang et al. [124] | ||
| Carbon nanotube | 10–20 nm diameter; 1–2 μm axial dimension | Short and tube | 0.1 wt.% | Sonication for 2 h | 30 min | Peng et al. [125] | |
| 90 nm diameter | Long rod like | 0.1, 0.3, 0.5, 0.7, and 1.0 wt.% | Stirring for 0.5, 1, 3 h | - | Sun et al. [126] | ||
| 20–30 nm in outer diameter; 10–30 µm in length | Pentagonal and heptagonal | 0.05, 0.10, 0.15, 0.20, and 0.25 wt.% | Proper stirring | 12 days | Min et al. [127] | ||
| SWCNTs (2 nm diameter), MWCNTs (25 ± 10 nm diameter) | Sphere | 50–100 μL | - | Few hours | Kristiansen et al. [128] | ||
| 8–50 nm in diameter, 0.5–30 µm in length | - | - | Magnetic stirring and ultrasonication for 2 h | 168 h | Ye et al. [129] | ||
| Carbon dots | CDs-IL 4.4 nm | Spherical | 3, 12.2, 34.9, 19.4 wt.% | Magnetic stirring for 6 h | 60 min | Tang et al. [130] | |
| Sulphur doped CQDs 4.8 nm | Spherical | 0.25, 1.25, 2.5, 5, and 10 wt.% | Ultrasonication for 30 min | 7 days | Xiao et al. [131] | ||
| CDs-GO 3–4 nm | - | 0.06, 0.08, 0.1, 0.2, 0.3 mg/mL | - | 6 months | Hu et al. [66] | ||
| Graphene | 1 nm | - | 23.8, 69.9, and 110 mg/mL | Magnetic stirring for 12 h | 1 month | Liang et al. [67] | |
| 100 nm | 2D nanosheet | 0.2 mg/mL | Stirring for 4 h | - | Fan et al. [132] | ||
| Size several micrometres, interlayer spacing 0.63 nm | Crystal | 0.5, 1.0, 1.5, 2.0, and 2.5 mg/mL | Ultrasonication for 30 min | - | Ma et al. [133] | ||
| 0.67–0.87 nm | Multiple layered | 0, 0.5, 1, 2, and 4 mg/mL | Stirring for 4 h, ultrasonication 8 h | - | Ye et al. [134] | ||
| 2 nm | - | 0.5, 1.5, 2.5, 4, 5, and 8 mg/mL | - | - | Qiang et al. [135] | ||
| - | Flat flake | 0.1, 1 wt.% | - | 30 days | Piatkowska et al. [136] | ||
| Diamond | 3–10 nm | spherical | 0.1, 0.5, 1, 2, 4, and 6 wt.% | Probe sonication, stirring | - | Mirzaamiri et al. [137] | |
| 5–10 nm | - | 0.01−0.07 wt.% | Simple stirring | - | Jiao et al. [68] | ||
| Graphene oxide | 1.20 & 1.45 nm | Sheet | 0, 0.3, 0.5, and 1 mg/mL | Ultrasonication for 30 min | 1 week | Fan et al. [73] | |
| 10–50 μm thick, 0.335 nm high | Single monolayer | 0.01 wt.% | Ultrasonication for 5 min | - | Kinoshita et al. [138] | ||
| 4 nm | - | 0–2 wt.% | Ultrasonication | - | Elomaa et al. [139] | ||
| 200–1000 nm | Transparent nanosheet | 0.1, 0.3, 0.5, 0.7, 1 wt.% | Ultrasonication | 12 days | Min et al. [72] | ||
| 0.5–5 μm diameter; 0.8–1.2 nm thick | - | 0.01, 0.05, 0.1, and 0.5 wt.% | Sonication for 2 h | - | Singh et al. [140] | ||
| 500 nm–5 μm diameter; 0.8–1.2 nm thick | Ultra-thin | 0.025, 0.05, 0.075, and 0.1 vol.% | Ultrasound, stirring | 3 months | Bai et al. [141] | ||
| 20–30 nm outer diameters; 10–30 µm length | 2D sheet | 0.5 mg/mL | Stirring for 30 min | 5 weeks | Song et al. [70] | ||
| 0.335 nm thick | Ultrathin and transparent | 0.8, 1.2, and 1.6 mg/mL | Stirring for 24 h, ultrasonication | 2 weeks | Gan et al. [142] | ||
| 10–50 μm lateral size; 1–2 nm thick | Spherical | 0.06 wt.%, 0.5 wt.% | Stirring for 30 min, ultrasonic bath for 10 min | 7 days | He et al. [17] | ||
| 2–5 nm thick 10–20 μm lateral size | - | 0.1 wt.% | Stirring for 30 min ultrasonic bath for 20 min | 50 days | Meng et al. [76] | ||
| 1 nm thick | initially sheet shape, then parabolic shape | 0.2 mg/mL | Sonication | - | Kim et al. [143] | ||
| 1.3 nm | Thin film | 0.05 to 1.0 mg/mL | Mechanical stirring for 30 min | 8 months | Hu et al. [75] | ||
| 0.8 μm lateral size 1.96 nm thick | Bathtub | 0, 0.03, 0.05, 0.07, and 0.1 wt.% | Magnetic stirring for 12 h, ultrasonication for 1 h | 90 days | Liu et al. [74] | ||
| GO & carbon | C (30–60 nm) and GO (30–60 nm) | C onion-like spherical; GO 2D nanosheet | C 0.06 wt.%; GO 0.02–0.06 wt.% | - | - | Su et al. [80] | |
| oxidised wood-derived nano carbons 640–1300 nm and GO 50–200 nm | aggregated chain-like | 0.001 and 1 wt.% | Ultrasonication for 30 min | 1 month | Kinoshita et al. [144] | ||
| GO & chitosan | GO 0.05–0.2 μm | GO optical 3D; copolymer brush-like | 2 mg/mL | Stirring for 6, 12 h ultrasonication | 30 days | Wei et al. [145] | |
| GO & 3-APS | 3-APS (525.39 nm) | - | 2 mg/mL | Stirring for a certain period | - | Li et al. [146] | |
| GO & graphene | GO 4.2 nm, graphene 5 nm | Multi-layered | 0.2, 0.5, 0.7 and 1.0 wt.% | Stirring for 1 h, ultrasonic bath 2 h | - | Xie et al. [77] | |
| GO & diamond | GO 2.5 nm and nanodiamond 2–10 nm | GO laminar | 0, 0.2, 0.4, 0.6, 0.8, 1.0 wt.% | Magnetic stirring for 9 h | - | Wu et al. [78] | |
| GO 30 nm, 2–3 nm thick; modified diamond 30 nm | GO lamellar and MD 3D structure | GO colloid (0.7 wt.%) and MD colloid (0.5 wt.%) | Ultrasonic ethanol bath for 5 min | 2 months | Liu et al. [79] | ||
| GO & graphitic CN | graphitic carbon nitride and GO 10–50 µm lateral size, 1–2 nm thick | unique one-layer | 0.06 wt.% each | Stirring for 30 min, ultrasonic bath for 10 min | - | He et al. [18] | |
| PEGlated graphene | 20 nm | laminar | 0.005, 0.01, 0.03, 0.05, and 0.1 wt.% | Mechanical stirring for 3, 4 h | 7 days | Hu et al. [147] | |
| Polymers | Cellulose | Length 200 ± 25 nm, Size 1–50 μm | Chain like, crystalline | 1, 1.5, 2, 2.5, 3, and 4 wt.% | - | - | Shariatzadeh et al. [148,149] |
| Fullerene–styrene and –acrylamide | 3–40 nm | Ideal spherical | 0.5 wt.% | Lei et al. [150] | |||
| average 46 nm | Ideal spherical | 0, 0.2, 0.4, 0.6 & 0.8 wt.% | - | - | Jiang et al. [151] | ||
| Hydrogel | - | Fibrous-3D network | 3, 4 & 5 wt.% | Stirring for 3–4 h, mechanical sheared | - | Wang et al. [152] | |
| Naphthalene | - | - | 0.02, 0.04, 0.06, 0.08, 0.1, 0.15, 0.2 mol/L | Stirring for 24 h | - | Yang et al. [153] | |
| Metal salts | LaF3 | LaDTP-10 (19.6 nm) and LaDTP-20 (8.5 nm) | LaDTP-10 polycrystalline; LaDTP-20 sphere | 1 wt.% | Continuous magnetic stirring for 1 h | - | Zhang et al. [154] |
| Proton type-ionic liquids | - | Chain like | 0, 0.25, 0.5, 0.75 & 1 wt.% | Stirring for 2 h | Zheng et al. [155] | ||
| - | Bilayered | 1 wt.% | Stirring for 12 h | - | Dong et al. [156] | ||
| - | brushy-like soft layer | 0.1 & 1 wt.% | Magnetic stirring for 2 h, ultrasonication for 10 min | 60 min | Khanmohammadi et al. [157] | ||
| - | - | 1 wt.% | Magnetic stirring for 10 min | - | Kreivaitis et al. [158] | ||
| Nitrides | Hydroxylated boron nitride (HO-BNNS) | 0.6–0.8 nm | Thin flat | HO-BNNS/water-glycol (0.0125, 0.025, 0.05, 0.10, 0.20 wt.%) | Ultrasonic process for 30 min | 5 days | Bai et al. [89] |
| Hexagonal boron nitride | 76.14 nm | - | 0.2 to 1.0 wt.% | Ultrasonication | 7 days | He et al. [159] | |
| - | - | 0.1–5.0 vol.% | - | - | Abdollah [160] | ||
| 300 nm wide and 30 nm thick | - | 1, 0.05 or 0.01 wt.% | Sonicator bath for 20 h | 30 days | Cho et al. [88] | ||
| Silicon nitride | Silica 20, 50, 100, 200 nm | - | - | - | - | Lin et al. [161] | |
| Carbides | Nb2C | 20 nm (Nb2C), 12 nm (MO-Nb2C), 6 nm (CO-Nb2C) | Accordion like, Crystalline | 1.0, 0.75, 0.5, and 0.25 mg/mL | Magnetic stirring for 6, 12 h, 7 days; ultrasonic stirring | CO-Nb2C 15 days; MO-Nb2C 30 days | Cheng et al. [90] |
| Ti3C2 | Lateral size 0.2–3 µm Layer thick 20 nm | Layered, Planar | 1, 2, 3, 5 and 7 wt.% | Magnetic stirring for 1 h | - | Nguyen et al. [91] | |
| Others | Black phosphorus | 3.9 nm | Crystalline | 0.001–0.02 wt.% | Ultrasonication for 8 h | 2 weeks | Tang et al. [92] |
| 500 nm | Honey-comb | 91.17% (wt.%) | Ultrasonication for 10 h | - | Wang et al. [162] | ||
| 100 nm wide; 7 nm thick | Multilayered | 35, 70, and 200 mg/L | Stirring for 10 min, ultrasonication | - | Wang et al. [163] | ||
| LDH | 19.73 nm wide; 8.68 nm thick | - | 0.5 wt.% | Ultrasonication | - | Wang et al. [94] | |
| 19.42 nm wide; 8.59 nm thick | Layered | 0.1–1.0 wt.% | Stirring for and ultrasonication | - | Wang et al. [93] | ||
| Chitosan | 70–145 nm | Crystalline | 0–0.5 wt.% | Ultrasonication for 15 min | 30 days | Li et al. [95] | |
| Stearic acid | - | 2D layered | 0.25, 0.5, 0.75 & 1.0 mg/mL | Ultrasonication | - | Ye et al. [96] |
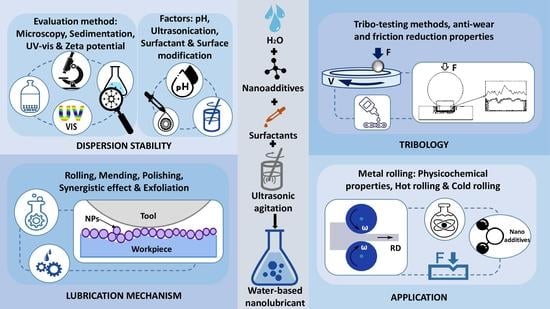
3. Dispersion Stability of Nanoadditives
3.1. Evaluation Methods
3.1.1. Microscopy
3.1.2. Zeta Potential Test
3.1.3. UV-Vis Spectral Analysis
3.1.4. Dynamic Light Scattering
3.1.5. Sedimentation
3.1.6. Other Methods
3.2. Factors Affecting Dispersion Stability
3.2.1. pH Control
3.2.2. Ultrasonication
3.2.3. Surface Modification
3.2.4. Surfactant Addition
| NPs Type | Surface Modifier | Surfactant |
|---|---|---|
| Pure metals | Bis (2-hydroxyethyl) dithiocarbamic acid (HAD) [46], Methoxylpolyethyleneglycol xanthate potassium (MPEGOCS2K) [47] | Polyvinylpyrrolidone (PVP) [46,97] |
| Metal and non-metal oxides | polyethylene glycol-200 [59], oleic acid (OA) [102], polyethyleneimine (PEI) [13,15,26,27,28], sodium hexametaphosphate (SHMP) [50], KH-570 [103] | (3-mercaptopropyl)trimethoxysilane (MPS) [85], sorbitan monostearate [53], polyvinylpyrrolidone (PVP) [54,97], polyethylene glycol (PEG) [54], cetrimonium bromide (CTAB), and sodium dodecylbenzene sulfonate (SDBS) [24,25,51,52,99], sodium silicate [51,52], snailcool [24], hexadecyl trimethyl ammonium bromide (CTAB) [101] |
| Metal sulphides | Bis (2-hydroxyethyl) dithiocarbamic acid (HDA) [62], sodium oleate soap, triethanolamine oleate, fatty alcohol polyethylene glycol ether (MOA), polyethylene glycol octyl phenyl ether (OP-4) [110], | (3-mercaptopropyl) trimethoxysilane (MPS) [61], cetrimonium bromide (CTAB), and sodium dodecylbenzene sulfonate (SDBS) [111], oleic acid, triethanolamine [111], |
| Carbon-based materials | Dopamine methacrylamide (DMA) 2-methacryloyloxyethyl phosphorylcholine (MPC) [68], | humic acid (HA) [128], sodium dodecyl sulfate (SDS) [119,120], Triton X-100 (C34H62O11) [67] |
| Composites | hexadecyldithiophosphate (DDP) [117], 3-mercaptopropyl trimethoxysilane (MPTS) [116], polydopamine (PDA) [118], | sodium dodecyl sulfate (SDS) [124,125], polyvinylpyrrolidone (PVP) [119], Igepal CO-520 [117], cetrimonium bromide (CTAB), and sodium dodecylbenzene sulfonate (SDBS) [119,184] |
| Others | dialkyl polyoxyethylene glycol thiophosphate ester (DTP-10, DTP-20) [154], oleylamine [93] | benzalkonium chloride [90], sodium polyacrylate (PAAS) [159], SHMP (sodium hexametaphosphate), 1,4-butylene glycol [203], coconut diethanol amide (CDEA) [204] |
3.3. Theories of Dispersion Stability
4. Tribo-Testing Methods
4.1. Four-Ball
4.2. Pin-on-Disk
4.3. Ball-on-Disk
4.4. Ball-on-Plate
4.5. Ball-on-Three-Plates
4.6. Block-on-Ring
4.7. Others
| Tribometer | Nanoparticle | Test Parameters | Testing Results | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Force | Speed | Temp. & Duration | Wear Reduction | Friction Reduction | Optimum Concentration | |||
| Four-balls | hBN | 100 N | 120–440 rpm | Room temp., 30 min | 95.73% | 60% | 0.05 wt.% | Bai et al. [89] |
| Capped Cu | - | 1450 rpm | 25 °C, 30 min | - | - | - | Zhang et al. [47] | |
| Cu-SiO2 | 50 N | 1450 rpm | 25 °C, 30 min | 37% | - | 1 wt.% | Zhang et al. [116] | |
| hBN | 392 N | 1200 rpm | 25 °C, 30 min | 14.6% | 29.1% | 0.7 wt.% | He et al. [159] | |
| Novel C | 0–7200 N | 500 rpm | 25 °C, 18 s | 96% | 76% | 1.2–2.0 wt.% | Peña-Parás [65] | |
| MWCNT | - | 1450 rpm | Room temp., 30 min | - | - | - | Peng et al. [125] | |
| CDs-IL | 30–80 N | 600 rpm | Room temp., 30 min | 64% | 57.5% | 0.015 wt.% | Tang et al. [130] | |
| Fe3O4-MoS2 | 294 N | 0.479 m/s | 30 min | 29.7% | 34.6% | - | Zheng et al. [86] | |
| LaF3 | 100–900 N | 1450 rpm | 20 °C, 30 min | - | - | 0.75–1 wt.% | Zhang et al. [154] | |
| fullerene–styrene | 200 N | 1450 rpm | 20 °C, 30 min | - | - | - | Lei et al. [150] | |
| fullerene–acrylamide | 200 N | 1450 rpm | 20 °C, 30 min | - | - | - | Jiang et al. [151] | |
| GO-TiO2 | 392 N | 1200 rpm | 20 °C, 30 min | - | - | 0.5 wt.% | Du et al. [84] | |
| MoO3 | 392 N | 1200–1760 rpm | 1800 s | - | - | 0.4 wt.% | Sun et al. [56] | |
| MoS2 and MoO3 | 392 N | 1200–1760 rpm | 1800 s | - | - | 0.3–0.5 wt.% | Meng et al. [63] | |
| Multilayer-MoS2 | 588 N | 1200 rpm | 30 days | - | - | - | Zhang et al. [111] | |
| SiO2 | - | 1760 rpm | Room temp., 10 s | - | - | 0.3 wt.% | Bao et al. [59] | |
| Dual-Coated TiO2 | 147 N | 1440 rpm | - | 34.8% | 0.17% | 1.6 wt.% | Gu et al. [103] | |
| OA–TiO2 | - | 1450 rpm | 25 °C, 30 min | - | - | 0.5 wt.% | Gao et al. [102] | |
| Nano-TiO2 | 196 N | 60 rpm | 30 min | 30.6% | 64.9% | 0.7 wt.% | Sun et al. [50] | |
| Nano-TiO2 | 200 N | 1200–1450 rpm | Room temp., 30 min | - | - | 0.5 wt.% | Kong et al. [49] | |
| Nano-TiO2 | 392 N | 1200–1760 rpm | 30 min | 47.4% | 33.8% | - | Meng et al. [52] | |
| Eu doped | 392 N | - | 60 min. | 0.62–0.37 mm | 0.083–0.065 | 0.5 wt.% | Liang et al. [123] | |
| Eu | - | - | 45–55 °C, 2 h | 0.62–0.35 mm | 0.083–0.055 | 0.6 wt.% | Xiong et al. [57] | |
| Pin-on-disk | hBN | 400–600 N | 300 rpm | 25 °C, 30 min | 14.6% | 29.1% | 0.7 wt.% | He et al. [159] |
| Ceria | - | 50 mm/s | Room temp., 30 min | 49% | 20% | 0.05–0.2% | Zhao et al. [53] | |
| Cr2O3 | 20–150 N | 50 mm/s | - | - | - | - | Cheng et al. [223] | |
| GO | 10 N | 0.02 m/s | 21–23 °C, 30 min | - | 57% | 1 wt.% | Elomaa et al. [139] | |
| Two phase fluids | 20 N | 100 rpm | 22 °C | - | ~0.05 | - | Pawlak et al. [224] | |
| Ball-on-disk | Ag-C | 1–9 N | 100–500 rpm | Room temp., 30 min | 40.4% | 80.6% | 1.0 wt.% | Song et al. [121] |
| Polyalkylene Glycol | 3 N | 24 mm/s | Room temp. | - | Around 20% | 0.5 wt.% | Wang et al. [94] | |
| Al2O3 (also disk on ball) | 4–10 N | 10–40 mm/s | - | 40–50% | 40–50% | - | Radice and Mischler [48] | |
| C dots | 10 N | - | Room temp., 1 h | 38% | 39.66% | Hu et al. [66] | ||
| CQD | 2 N | 150 cycles/min | Room temp., 12 min | - | 30% | - | HuaPing et al. [131] | |
| Urea modified C | 3–7 N | 200–400 rpm | 30 min | 96.70% | 80.86% | 0.15 wt.% | Min et al. [127] | |
| Hexagonal BN | 5.64 N | 10.2 mm/s | Room temp, 30 days | - | - | - | Cho et al. [88] | |
| DDP-Cu | 1–4 N | - | 25 °C, 30 min | 60.5% | 45.5% | 0.2–0.4 wt.% | Liu et al. [117] | |
| Diamond | - | 80 mm/s | 30 °C | 88% | 70% | 2 wt.% | Mirzaamiri [137] | |
| γ-Fe2O3 | 4 N | 0.20 m/s | Room temp. | - | - | 0.6 wt.% | Pardue et al. [55] | |
| GO/Chitosan | 100 N | - | - | 47% | 84% | - | Wei et al. [145] | |
| Graphene quantum dots | 100 N | - | Room temp., 60 min | 58.5% | 42.5% | - | Qiang et al. [135] | |
| GO-MoS2 | 0.5–3 N | 60 rpm | 25 °C | - | 50% | - | Liu et al. [225] | |
| FGO | 5 N | 300 r/min | 30 min | 88.1% | 41.4% | 0.7 wt.% | Min et al. [72] | |
| GO | 5–20 N | 0.005–0.1 m/s | Room temp | 68% | 78.5% | 0.1 wt.% | Singh et al. [140] | |
| GO-OLC | 2–10 N | 200 rpm | Room temp | - | - | 0.06 wt.% | Su et al. [80] | |
| Nanofilm GO | 2 N | 12 mm/s | 25 °C, 60 min | 79.7% | 43.6% | - | Li et al. [146] | |
| SiO2-GO | 10 N | - | 25–35 °C | 78.3% | - | 0.05 wt.% | Guo et al. [83] | |
| PEGlated graphene | 10 N | - | 30 min | 81.23% | 39.04% | 0.05 wt.% | Hu et al. [147] | |
| Hydroxide | 2N | 0.024 m/s | 25 °C. 45 min | 43.2% | 83.1% | 0.5 wt.% | Wang et al. [93] | |
| Al2O3-WS2-MoS2 | 10 N | 320 rpm | Amb. Temp. | 23.4% | 53.89% | - | Kumar et al. [119] | |
| Black phosphorus | 10–70 N | - | - | 97.1% | 25% | - | Wang et al. [162] | |
| BP | 8–15 N | 150 r/min | 30 min | 61.1% | 32.4% | - | Wang et al. [163] | |
| Si3N4 | 15, 30, 60 N | 0.25 m/s, 0.5 m/s | 27 °C, 3600 s | - | - | - | Lin et al. [161] | |
| Ti3C2 | 3–10 N | 120 rpm, 0.126 m/s | 24–26 °C, 1 h | 48% | 20% | 5 wt.% | Nguyen and Chung [91] | |
| TiO2 | 5 N | 50 mm/s | 25 °C, 30 min | - | 16.3% | 0.4–8.0 wt.% | Wu et al. [14] | |
| TiO2 | 20–80 N | 50 mm/s | 10 min | 70.5% | 84.3% | 4 wt.% | Wu et al. [25] | |
| NaCl saline | 10–100 | 50 mm/s | 1 h | - | - | 3.5 wt.% | Wu et al. [226] | |
| ZnO and Al2O3 | 10 N | 100 mm/s | - | - | 56.9% | - | Gara and Zou [122] | |
| Ceramics | 30 N | 0.5 m/s | Room temp., 3600 s | 54.0% | 78.8% | - | Cui et al. [109] | |
| Chitosan | 5–30 N | 12–36 mm/s | 25 °C, 30 min | 69% | 40% | 0.3 wt.% | Li et al. [95] | |
| Individual additives | 3 N | 20 mm/s | Room temp. 1 h | - | 12%, 30% | 0.05%, 0.1% | Tomala et al. [203] | |
| Ball-on-plate | Hard C microsphere | 100–300 mN | 10 mm/s | 30 min | - | - | 0.1 wt.% | Wang et al. [124] |
| Cu | 1–4 N | 0.02 m/s | 22 °C, 30 min | 85–99.9% | 80.6% | 0.6 wt.% | Zhao et al. [46] | |
| CuO | - | 20 mm/s | 22 °C, 30 min | 72.6–89.1% | 43.2–52.2% | 0.8 wt.% | Zhao et al. [54] | |
| Nano diamond | 1 N | 360 rpm | 25 °C, 30 min | - | 40% | - | Jiao et al. [68] | |
| Graphene and GO | 1–8 N | 0.08 m/s | 30 min | 13.5% | 21.9% | 0.5 wt.% | Xie et al. [77] | |
| Fluorinated GO | 20 N | 4 mm/s | Room temp., 2000 s | 47% | - | - | Fan et al. [73] | |
| MGO | 5–25 N | - | Room temp., 3000 s | 74% | - | - | Gan et al. [142] | |
| GO-ND | 0–1 N | 0.4 mm/s | 25 °C, 1800 s | - | - | 0.1 wt.% GO, 0.5 wt.% ND | Wu et al. [78] | |
| GO-MD | - | - | 250 s | - | 0.6–0.01 | 0.7 wt.% GO, 0.5 wt.% MD | Liu et al. [79] | |
| Graphene water-based | 10 N | 0.01 m/s | - | - | - | 0.1 wt.% graphene flakes. 1% wt.% graphite | Piątkowska et al. [136] | |
| Graphene-SiO2 | 3 N | 0.08 m/s | Room temp., 30 min | 79% | 48.5% | 0.5 wt.% | Xie et al. [112] | |
| Monolayer GO | 1.88 N | 0.5 mm/s | - | Marginal after 60,000 cycles | ~0.05 after 60,000 cycles | 0.01 wt.% | Kinoshita et al. [138] | |
| Oxide graphene | 10 N | 120 rpm | 10 min | - | - | <0.1 wt.% | Song and Li [70] | |
| Metal doped CDs | 40–500 N | - | 20–120 min | Up to 43.1% | Up to 73.5% | 1.0 wt.% | Tang et al. [227] | |
| Reduced GO | 50–200 N | 4mm/s | - | 70 μm after 100,000 cycles | Around 0.1 after 100,000 cycles | 0.01 wt.% | Kim and Kim [143] | |
| PEI-RGO | - | 9000 r/min | - | 45% | 54.6% | 0.05 wt.% | Liu et al. [74] | |
| Protic ionic (PILs) | 2–4 N | - | 30 °C, 30 min | 85% | 80% | 1 wt.% | Kreivaitis et al. [158] | |
| MoS2 | 20 N | - | 25 °C | - | - | 0.1 wt.% | Wang et al. [64] | |
| Naphthalene | 100 N | 1475 rpm | 30 min | - | - | - | Yang et al. [153] | |
| BPQDs | 40–300 N | 10 mm/s | 30 °C, 20–120 min | 56.4% | 32.3% | 0.005 wt.% | Tang et al. [92] | |
| CNT/SiO2 | 5 N | 120 rpm | 10 min | - | 66.4% | 0.5 wt.% | Xie et al. [113] | |
| rGO | 20 mN | 4 mm/s | - | - | 12 times | 5 μL/min | Kim et al. [228] | |
| Ball-on-three-plates | Alumina | 10–40 N | 20 to 100 mm/s | 10 min | 22% | 27% | 2 wt.% | He et al. [16] |
| g-C3N4/GO | 10–35 N | 25 to 125 mm/s | 25 °C | 19.6% | 37% | 0.06 wt.% | He et al. [18] | |
| pH-GO | 20 N | 50 mm/s | - | 17.1% | 44.4% | 0.06 wt.% | He et al. [17] | |
| MR fluid | 0.5 N | 1.18 m/s | 2–10 min | - | - | 1 vol% | Rosa et al. [229] | |
| Block-on-ring | Novel C | 245 N | 300 rpm | 1200 s | 96% | 76% | 2 wt.% | Peña-Parás [65] |
| GO-Al2O3 | 10 to 30 N | 100 to 400 mm/s | 20–25 °C, last 7 min | - | 47–64% | 0.06 wt.% | Huang et al. [21] | |
| ZrO2/TiO2 | 100 N | 400 mm/s | - | 65% | 25% | - | Huang et al. [12] | |
| GO-SiO2 | 20 N | 109 rpm | Ambient Temp. | - | - | 0.16 wt.% | Huang et al. [19] | |
| Ring-on-plate | Alkyl glucopyranosides (AGPs) | 50 N | 0.1 m/s | Room temp, 1 h | - | >95% | - | Chen et al. [222] |
| Ball-on-block | MWCNT | 50 N | - | 30 min | 66% | - | - | Ye et al. [129] |
| urea-modified FG | Ye et al. [134] | |||||||
| Stearic acid | - | - | 30–500 °C | 57–90% | 68–83% | - | Ye et al. [96] | |
| Piston ring-on-cylinder | Cellulose | 50 N | 130–300 rpm | Room temp. | >50% | ~75% | 2 wt.% | Shariatzadeh and Grecov [148,149] |
| 2 ball-plate | Px-CNTs | 5 N | 120 rpm | 10 min | - | 66.4% | 0.5 wt.% | Sun et al. [126] |
5. Lubrication Mechanism
5.1. Rolling/Ball Bearing Effect
5.2. Protective Film/Tribo-Film
5.3. Mending Effect
5.4. Polishing/Smoothing Effect
5.5. Synergistic Effect
5.6. Exfoliation
5.7. Hydration Lubrication
6. Application of Water-Based Nanolubricants in Metal Rolling
6.1. Physicochemical Properties of Applied Lubricants
6.2. Hot Rolling of Steels
6.2.1. Rolling Force
6.2.2. Surface Morphology of Rolled Steel
6.2.3. Oxidation Behaviour of Steel
6.2.4. Microstructure of Rolled Steel
6.3. Cold Rolling of Steels
6.4. Cold Rolling of Non-Ferrous Metals
7. Conclusions and Outlook
- Ensuring long-term dispersion stability of nanoadditives in water is still a big challenge. The interaction among different lubricant components needs to be investigated for the perfection of the theories of dispersion stability.
- For the application in metal rolling, the formulation of water-based nanolubricants needs to be optimised to further enhance their physicochemical properties in terms of dispersion stability, wettability, and extreme pressure property. Special attention should be given to the strategies for reducing material and preparation costs of the applied nanolubricants.
- The application of water-based nanolubricants in hot steel rolling has exhibited positive effects on the decreases in rolling force, rolled surface roughness, and oxide scale thickness, and also enabled refined grains in microstructure. However, the lubrication effects on controls of profile, flatness, and texture have been rarely involved. More studies are also needed to examine the grain refinement mechanism and attain maximally refined grains, which is a promising and economical technique to significantly promote the overall properties of hot rolled steels.
- For the case of application of cold steel rolling, it is of vital importance to have more focus on the study of the corrosive property of applied water-based nanolubricants. In addition to the lubrication effects on rolling force and surface quality, extra attention should be paid to those on rolling texture and shape control.
- Although certain water-based nanolubrication mechanisms in rolling of steels have been proposed through analysis of post-rolling specimen by means of electron microscopy, in situ observation of NPs and demonstration of their motion behaviour have not been specifically conducted. To have a systematic and comprehensive understanding of the lubrication mechanisms, varying rolling parameters such as rolling temperature, rolling reduction, and speed should be employed, and corresponding multi-scale numerical simulation can be carried out.
- As pointed out earlier, work roll service life can be prolonged using water-based nanolubricants, which largely reduces the roll changing frequency and thus enhances the productivity of rolling mill. However, no research has been conducted to quantitatively evaluate the wear of work rolls under water-based nanolubrication conditions.
- The use of green lubricant is becoming mainstream in sustainable manufacturing. It is of vital importance to develop a cost-effective recycling technology for waste water-based nanolubricants.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhushan, B.; Israelachvili, J.N.; Landman, U. Nanotribology-Friction, Wear and Lubrication at the Atomic-Scale. Nature 1995, 374, 607–616. [Google Scholar] [CrossRef]
- Fu, Y.; Batchelor, A.W.; Loh, N.K.; Tan, K.W. Effect of lubrication by mineral and synthetic oils on the sliding wear of plasma nitrided AISI 410 stainless steel. Wear 1998, 219, 169–176. [Google Scholar] [CrossRef]
- Haus, F.; German, J.; Junter, G.-A. Primary biodegradability of mineral base oils in relation to their chemical and physical characteristics. Chemosphere 2001, 45, 983–990. [Google Scholar] [CrossRef]
- Sotres, J.; Arnebrant, T. Experimental Investigations of biological lubrication at the nanoscale: The cases of synovial joints and the oral cavity. Lubricants 2013, 1, 102–131. [Google Scholar] [CrossRef] [Green Version]
- Dubey, S.; Sharma, G.; Shishodia, K.; Sekhon, G. Study on the performance of oil-in-water emulsions during cold rolling of steel strip. Tribol. Trans. 2005, 48, 499–504. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Jing, H. Application of oil-in-water emulsion in hot rolling process of brass sheet. Ind. Lubr. Tribol. 2010, 62, 224–231. [Google Scholar] [CrossRef]
- Xia, W.; Zhao, J.; Wu, H.; Jiao, S.; Zhao, X.; Zhang, X.; Xu, J.; Jiang, Z. Analysis of oil-in-water based nanolubricants with varying mass fractions of oil and TiO2 nanoparticles. Wear 2018, 396–397, 162–171. [Google Scholar] [CrossRef]
- Xia, W.Z.; Zhao, J.W.; Wu, H.; Jiao, S.H.; Jiang, Z.Y. Effects of oil-in-water based nanolubricant containing TiO2 nanoparticles on the tribological behaviour of oxidised high-speed steel. Tribol. Int. 2017, 110, 77–85. [Google Scholar] [CrossRef]
- Xia, W.Z.; Zhao, J.W.; Wu, H.; Zhao, X.M.; Zhang, X.M.; Xu, J.Z.; Jiao, S.H.; Wang, X.G.; Zhou, C.L.; Jiang, Z.Y. Effects of oil-in-water based nanolubricant containing TiO2 nanoparticles in hot rolling of 304 stainless steel. J. Mater. Process. Technol. 2018, 262, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Renner, P.; Liang, H. Dispersion of Nanoparticles in Lubricating Oil: A Critical Review. Lubricants 2019, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Gulzar, M.; Masjuki, H.H.; Kalam, M.A.; Varman, M.; Zulkifli, N.W.M.; Mufti, R.A.; Zahid, R. Tribological performance of nanoparticles as lubricating oil additives. J. Nanopart. Res. 2016, 18, 223. [Google Scholar] [CrossRef]
- Huang, S.Q.; Lin, W.K.; Li, X.L.; Fan, Z.Q.; Wu, H.; Jiang, Z.Y.; Huang, H. Roughness-dependent tribological characteristics of water-based GO suspensions with ZrO2 and TiO2 nanoparticles as additives. Tribol. Int. 2021, 161, 107073. [Google Scholar] [CrossRef]
- Wu, H.; Jia, F.H.; Zhao, J.W.; Huang, S.Q.; Wang, L.Z.; Jiao, S.H.; Huang, H.; Jiang, Z.Y. Effect of water-based nanolubricant containing nano-TiO2 on friction and wear behaviour of chrome steel at ambient and elevated temperatures. Wear 2019, 426, 792–804. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, J.W.; Cheng, X.W.; Xia, W.Z.; He, A.S.; Yun, J.H.; Huang, S.Q.; Wang, L.Z.; Huang, H.; Jiao, S.H.; et al. Friction and wear characteristics of TiO2 nano-additive water-based lubricant on ferritic stainless steel. Tribol. Int. 2018, 117, 24–38. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Zhao, J.W.; Xia, W.Z.; Cheng, X.W.; He, A.S.; Yun, J.H.; Wang, L.Z.; Huang, H.; Jiao, S.H.; Huang, L.; et al. A study of the tribological behaviour of TiO2 nano-additive water-based lubricants. Tribol. Int. 2017, 109, 398–408. [Google Scholar] [CrossRef] [Green Version]
- He, A.S.; Huang, S.Q.; Yun, J.H.; Wu, H.; Jiang, Z.Y.; Stokes, J.; Jiao, S.H.; Wang, L.Z.; Huang, H. Tribological Performance and Lubrication Mechanism of Alumina Nanoparticle Water-Based Suspensions in Ball-on-Three-Plate Testing. Tribol. Lett. 2017, 65, 40. [Google Scholar] [CrossRef]
- He, A.S.; Huang, S.Q.; Yun, J.H.; Jiang, Z.Y.; Stokes, J.; Jiao, S.H.; Wang, L.Z.; Huang, H. The pH-dependent structural and tribological behaviour of aqueous graphene oxide suspensions. Tribol. Int. 2017, 116, 460–469. [Google Scholar] [CrossRef] [Green Version]
- He, A.S.; Huang, S.Q.; Yun, J.H.; Jiang, Z.Y.; Stokes, J.R.; Jiao, S.H.; Wang, L.Z.; Huang, H. Tribological Characteristics of Aqueous Graphene Oxide, Graphitic Carbon Nitride, and Their Mixed Suspensions. Tribol. Lett. 2018, 66, 42. [Google Scholar] [CrossRef]
- Huang, S.Q.; Li, X.; Yu, B.; Jiang, Z.; Huang, H. Machining characteristics and mechanism of GO/SiO2 nanoslurries in fixed abrasive lapping. J. Mater. Process. Technol. 2020, 277, 116444. [Google Scholar] [CrossRef]
- Huang, S.Q.; Li, X.L.; Zhao, Y.T.; Sun, Q.; Huang, H. A novel lapping process for single-crystal sapphire using hybrid nanoparticle suspensions. Int. J. Mech. Sci. 2021, 191, 106099. [Google Scholar] [CrossRef]
- Huang, S.Q.; He, A.S.; Yun, J.H.; Xu, X.F.; Jiang, Z.Y.; Jiao, S.H.; Huang, H. Synergistic tribological performance of a water based lubricant using graphene oxide and alumina hybrid nanoparticles as additives. Tribol. Int. 2019, 135, 170–180. [Google Scholar] [CrossRef]
- Lin, W.; Kampf, N.; Klein, J. Designer Nanoparticles as Robust Superlubrication Vectors. ACS Nano 2020, 14, 7008–7017. [Google Scholar] [CrossRef]
- Lin, W.; Kampf, N.; Goldberg, R.; Driver, M.J.; Klein, J. Poly-phosphocholinated Liposomes Form Stable Superlubrication Vectors. Langmuir 2019, 35, 6048–6054. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Kamali, H.; Huo, M.; Lin, F.; Huang, S.; Huang, H.; Jiao, S.; Xing, Z.; Jiang, Z. Eco-Friendly Water-Based Nanolubricants for Industrial-Scale Hot Steel Rolling. Lubricants 2020, 8, 96. [Google Scholar] [CrossRef]
- Wu, H.; Jia, F.; Li, Z.; Lin, F.; Huo, M.; Huang, S.; Sayyar, S.; Jiao, S.; Huang, H.; Jiang, Z. Novel water-based nanolubricant with superior tribological performance in hot steel rolling. Int. J. Extrem. Manuf. 2020, 2, 025002. [Google Scholar] [CrossRef]
- Huo, M.; Wu, H.; Xie, H.; Zhao, J.; Su, G.; Jia, F.; Li, Z.; Lin, F.; Li, S.; Zhang, H.; et al. Understanding the role of water-based nanolubricants in micro flexible rolling of aluminium. Tribol. Int. 2020, 151, 106378. [Google Scholar] [CrossRef]
- Wu, H.; Jiang, C.Y.; Zhang, J.Q.; Huang, S.Q.; Wang, L.Z.; Jiao, S.H.; Huang, H.; Jiang, Z.Y. Oxidation Behaviour of Steel During hot Rolling by Using TiO2-Containing Water-Based Nanolubricant. Oxid. Met. 2019, 92, 315–335. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, J.W.; Luo, L.; Huang, S.Q.; Wang, L.Z.; Zhang, S.Q.; Jiao, S.H.; Huang, H.; Jiang, Z.Y. Performance Evaluation and Lubrication Mechanism of Water-Based Nanolubricants Containing Nano-TiO2 in Hot Steel Rolling. Lubricants 2018, 6, 57. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Zhao, J.W.; Xia, W.Z.; Cheng, X.W.; He, A.S.; Yun, J.H.; Wang, L.Z.; Huang, H.; Jiao, S.H.; Huang, L.; et al. Analysis of TiO2 nano-additive water-based lubricants in hot rolling of microalloyed steel. J. Manuf. Process. 2017, 27, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Zhou, J.; Jiang, Z. Developments and Possibilities for Nanoparticles in Water-Based Lubrication During Metal Processing. Rev. Nanosci. Nanotechnol. 2016, 5, 136–163. [Google Scholar] [CrossRef]
- Rahman, M.H.; Warneke, H.; Webbert, H.; Rodriguez, J.; Austin, E.; Tokunaga, K.; Rajak, D.K.; Menezes, P.L. Water-Based Lubricants: Development, Properties, and Performances. Lubricants 2021, 9, 73. [Google Scholar] [CrossRef]
- Canter, N. Special Report: Trends in extreme pressure additives. Tribol. Lubr. Technol. 2007, 63, 10. [Google Scholar]
- Choi, S.U.; Eastman, J.A. Enhanced Heat Transfer Using Nanofluids. U.S. Patent 6221275, 1 January 2001. [Google Scholar]
- Akoh, H.; Tsukasaki, Y.; Yatsuya, S.; Tasaki, A. Magnetic properties of ferromagnetic ultrafine particles prepared by vacuum evaporation on running oil substrate. J. Cryst. Growth 1978, 45, 495–500. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.-Z.; Zhu, J.-J.; Chen, H.-Y. Preparation of CuO nanoparticles by microwave irradiation. J. Cryst. Growth 2002, 244, 88–94. [Google Scholar] [CrossRef]
- Sandhya, S.U.; Nityananda, S.A. A Facile One Step Solution Route to Synthesize Cuprous Oxide Nanofluid. Nanomater. Nanotechnol. 2013, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Han, S.Y.; Shin, S.Y.; Lee, H.-J.; Lee, B.-J.; Lee, S.; Kim, N.J.; Kwak, J.-H. Effects of annealing temperature on microstructure and tensile properties in ferritic lightweight steels. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2012, 43, 843–853. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Mayers, B.; Herricks, T.; Xia, Y. Polyol Synthesis of Uniform Silver Nanowires: A Plausible Growth Mechanism and the Supporting Evidence. Nano Lett. 2003, 3, 955–960. [Google Scholar] [CrossRef]
- Eastman, J.A.; Choi, S.U.S.; Li, S.; Yu, W.; Thompson, L.J. Anomalously increased effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl. Phys. Lett. 2001, 78, 718–720. [Google Scholar] [CrossRef]
- Hsin, Y.L.; Hwang, K.C.; Chen, F.-R.; Kai, J.-J. Production and in-situ Metal Filling of Carbon Nanotubes in Water. Adv. Mater. 2001, 13, 830–833. [Google Scholar] [CrossRef]
- Lo, C.-H.; Tsung, T.-T.; Chen, L.-C. Shape-controlled synthesis of Cu-based nanofluid using submerged arc nanoparticle synthesis system (SANSS). J. Cryst. Growth 2005, 277, 636–642. [Google Scholar] [CrossRef]
- Angayarkanni, S.A.; Philip, J. Review on thermal properties of nanofluids: Recent developments. Adv. Colloid. Interface Sci. 2015, 225, 146–176. [Google Scholar] [CrossRef] [PubMed]
- Haddad, Z.; Abid, C.; Oztop, H.F.; Mataoui, A. A review on how the researchers prepare their nanofluids. Int. J. Sci. 2014, 76, 168–189. [Google Scholar] [CrossRef]
- Shahnazar, S.; Bagheri, S.; Abd Hamid, S.B. Enhancing lubricant properties by nanoparticle additives. Int. J. Hydrogen Energy 2016, 41, 3153–3170. [Google Scholar] [CrossRef]
- Liu, G.; Li, X.; Qin, B.; Xing, D.; Guo, Y.; Fan, R. Investigation of the mending effect and mechanism of copper nano-particles on a tribologically stressed surface. Tribol. Lett. 2004, 17, 961–966. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, G.; Zhang, C.; Zhang, Y.; Zhang, S.; Zhang, P. Synthesis of water-soluble Cu nanoparticles and evaluation of their tribological properties and thermal conductivity as a water-based additive. Friction 2019, 7, 246–259. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhang, S.; Song, S.; Yang, G.; Yu, L.; Wu, Z.; Li, X.; Zhang, P. Preparation and Tribological Properties of Surface-Capped Copper Nanoparticle as a Water-Based Lubricant Additive. Tribol. Lett. 2014, 54, 25–33. [Google Scholar] [CrossRef]
- Radice, S.; Mischler, S. Effect of electrochemical and mechanical parameters on the lubrication behaviour of Al2O3 nanoparticles in aqueous suspensions. Wear 2006, 261, 1032–1041. [Google Scholar] [CrossRef]
- Kong, L.; Sun, J.; Bao, Y.; Meng, Y. Effect of TiO2 nanoparticles on wettability and tribological performance of aqueous suspension. Wear 2017, 376–377, 786–791. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Xu, P.; Zhu, Z. Study on the lubricating performance of nano-TiO2 in water-based cold rolling fluid. Mater. Sci. Forum 2015, 3, 3988–3992. [Google Scholar]
- Zhu, Z.; Sun, J.; Wei, H.; Niu, T.; Zhu, Z. Research on Lubrication Behaviors of Nano-TiO2 in Water-Based Hot Rolling Liquid. Adv. Mater. Res. 2013, 643, 139–143. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, J.; Wu, P.; Dong, C.; Yan, X. The Role of Nano-TiO2 Lubricating Fluid on the Hot Rolled Surface and Metallographic Structure of SS41 Steel. Nanomaterials 2018, 8, 111. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Chen, Y.K.; Ren, G. A Study of Tribological Properties of Water-Based Ceria Nanofluids. Tribol. Trans. 2013, 56, 275–283. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, G.; Zhang, Y.; Zhang, S.; Zhang, C.; Gao, C.; Zhang, P. Controllable synthesis of different morphologies of CuO nanostructures for tribological evaluation as water-based lubricant additives. Friction 2020, 9, 963–977. [Google Scholar] [CrossRef]
- Pardue, T.; Acharya, B.; Curtis, C.; Krim, J. A Tribological Study of γ-Fe2O3 Nanoparticles in Aqueous Suspension. Tribol. Lett. 2018, 66, 130. [Google Scholar] [CrossRef]
- Sun, J.; Meng, Y.; Zhang, B. Tribological Behaviors and Lubrication Mechanism of Water-based MoO3 Nanofluid during Cold Rolling Process. J. Manuf. Process. 2021, 61, 518–526. [Google Scholar] [CrossRef]
- Xiong, S.; Liang, D.; Kong, F. Effect of pH on the Tribological Behavior of Eu-Doped WO3 Nanoparticle in Water-Based Fluid. Tribol. Lett. 2020, 68, 126. [Google Scholar] [CrossRef]
- Ding, M.; Lin, B.; Sui, T.; Wang, A.; Yan, S.; Yang, Q. The excellent anti-wear and friction reduction properties of silica nanoparticles as ceramic water lubrication additives. Ceram. Int. 2018, 44, 14901–14906. [Google Scholar] [CrossRef]
- Bao, Y.; Sun, J.; Kong, L. Tribological properties and lubricating mechanism of SiO2 nanoparticles in water-based fluid. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 182, p. 012025. [Google Scholar] [CrossRef] [Green Version]
- Kogovšek, J.; Remškar, M.; Mrzel, A.; Kalin, M. Influence of surface roughness and running-in on the lubrication of steel surfaces with oil containing MoS2 nanotubes in all lubrication regimes. Tribol. Int. 2013, 61, 40–47. [Google Scholar] [CrossRef]
- Kuznetsova, Y.; Rempel, S.V.; Popov, I.D.; Gerasimov, E.; Rempel, A. Stabilization of Ag2S nanoparticles in aqueous solution by MPS. Colloids Surf. Phys. Eng. Asp. 2017, 520, 369–377. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, G.; Zhang, Y.; Zhang, S.; Zhang, P. A Simple Preparation of HDA-CuS Nanoparticles and Their Tribological Properties as a Water-Based Lubrication Additive. Tribol. Lett. 2019, 67, 88. [Google Scholar] [CrossRef]
- Yanan, M.; Jianlin, S.; Jiaqi, H.; Xudong, Y.; Yu, P. Recycling prospect and sustainable lubrication mechanism of water-based MoS2 nano-lubricant for steel cold rolling process. J. Clean. Prod. 2020, 277, 123991. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Y.; Deng, J.; Wang, Z. Friction reduction of water based lubricant with highly dispersed functional MoS2 nanosheets. Colloids Surf. A Phys. Eng. Asp. 2019, 562, 321–328. [Google Scholar] [CrossRef]
- Peña-Parás, L.; Maldonado-Cortés, D.; Kharissova, O.V.; Saldívar, K.I.; Contreras, L.; Arquieta, P.; Castaños, B. Novel carbon nanotori additives for lubricants with superior anti-wear and extreme pressure properties. Tribol. Int. 2019, 131, 488–495. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Wang, C.; Ye, Y.; Zhao, H.; Li, J.; Lu, X.; Mao, C.; Chen, S.; Mao, J.; et al. One-pot pyrolysis preparation of carbon dots as eco-friendly nanoadditives of water-based lubricants. Carbon 2019, 152, 511–520. [Google Scholar] [CrossRef]
- Liang, S.; Shen, Z.; Yi, M.; Liu, L.; Zhang, X.; Ma, S. In-situ exfoliated graphene for high-performance water-based lubricants. Carbon 2016, 96, 1181–1190. [Google Scholar] [CrossRef]
- Jiao, Y.; Liu, S.; Sun, Y.; Yue, W.; Zhang, H. Bioinspired Surface Functionalization of Nanodiamonds for Enhanced Lubrication. Langmuir 2018, 34, 12436–12444. [Google Scholar] [CrossRef]
- Si, Y.; Samulski, E.T. Synthesis of Water Soluble Graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-J.; Li, N. Frictional behavior of oxide graphene nanosheets as water-base lubricant additive. Appl. Phys. A 2011, 105, 827–832. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Min, C.; He, Z.; Song, H.; Liang, H.; Liu, D.; Dong, C.; Jia, W. Fluorinated graphene oxide nanosheet: A highly efficient water-based lubricated additive. Tribol. Int. 2019, 140, 105867. [Google Scholar] [CrossRef]
- Fan, K.; Liu, J.; Wang, X.; Liu, Y.; Lai, W.; Gao, S.; Qin, J.; Liu, X. Towards enhanced tribological performance as water-based lubricant additive: Selective fluorination of graphene oxide at mild temperature. J. Colloid Interface Sci. 2018, 531, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Guo, Y.; Wang, D. PEI-RGO nanosheets as a nanoadditive for enhancing the tribological properties of water-based lubricants. Tribol. Int. 2019, 140, 105851. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Zeng, Z.; Zhao, H.; Li, J.; Ge, X.; Wang, L.; Xue, Q.; Mao, C.; Chen, S. BLG-RGO: A novel nanoadditive for water-based lubricant. Tribol. Int. 2019, 135, 277–286. [Google Scholar] [CrossRef]
- Meng, W.; Sun, J.; Wang, C.; Wu, P. pH-dependent lubrication mechanism of graphene oxide aqueous lubricants on the strip surface during cold rolling. Surf. Interface Anal. 2021, 53, 406–417. [Google Scholar] [CrossRef]
- Xie, H.; Jiang, B.; Dai, J.; Peng, C.; Li, C.; Li, Q.; Pan, F. Tribological Behaviors of Graphene and Graphene Oxide as Water-Based Lubricant Additives for Magnesium Alloy/Steel Contacts. Materials 2018, 11, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.; Chen, X.; Zhang, C.; Luo, J. Synergistic tribological behaviors of graphene oxide and nanodiamond as lubricating additives in water. Tribol. Int. 2019, 132, 177–184. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Pan, G.; Luo, J. A comparative study between graphene oxide and diamond nanoparticles as water-based lubricating additives. Sci. China Technol. Sci. 2013, 56, 152–157. [Google Scholar] [CrossRef]
- Su, F.; Chen, G.; Huang, P. Lubricating performances of graphene oxide and onion-like carbon as water-based lubricant additives for smooth and sand-blasted steel discs. Friction 2020, 8, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.-T.; Lee, J.-S.; Sekino, T.; Niihara, K. Fabrication of Cu dispersed Al2O3 nanocomposites using Al2O3/CuO and Al2O3/Cu-nitrate mixtures. Scr. Mater. 2001, 44, 2117–2120. [Google Scholar] [CrossRef]
- Devendiran, D.K.; Amirtham, V.A. A review on preparation, characterization, properties and applications of nanofluids. Renew. Sustain. Energy Rev. 2016, 60, 21–40. [Google Scholar] [CrossRef]
- Guo, P.; Chen, L.; Wang, J.; Geng, Z.; Lu, Z.; Zhang, G. Enhanced Tribological Performance of Aminated Nano-Silica Modified Graphene Oxide as Water-Based Lubricant Additive. ACS Appl. Nano Mater. 2018, 1, 6444–6453. [Google Scholar] [CrossRef]
- Du, S.; Sun, J.; Wu, P. Preparation, characterization and lubrication performances of graphene oxide-TiO2 nanofluid in rolling strips. Carbon 2018, 140, 338–351. [Google Scholar] [CrossRef]
- Li, W.; Zou, C.; Li, X. Thermo-physical properties of cooling water-based nanofluids containing TiO2 nanoparticles modified by Ag elementary substance for crystallizer cooling system. Powder Technol. 2018, 329, 434–444. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, Y.; Geng, J.; Peng, Y.; Olson, D.; Hu, X. Tribological behavior of Fe3O4/MoS2 nanocomposites additives in aqueous and oil phase media. Tribol. Int. 2016, 102, 79–87. [Google Scholar] [CrossRef]
- Lelonis, D.A.; Tereshko, J.W.; Andersen, C.M. Boron Nitride Powder—A High-Performance Alternative for Solid Lubrication. GE Adv. Ceram. 2003, 4, 81506. [Google Scholar]
- Cho, D.-H.; Kim, J.-S.; Kwon, S.-H.; Lee, C.; Lee, Y.-Z. Evaluation of hexagonal boron nitride nano-sheets as a lubricant additive in water. Wear 2013, 302, 981–986. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, L.; Ge, C.; Liu, R.; Guan, H.; Zhang, X. Atomically Thin Hydroxylation Boron Nitride Nanosheets for Excellent Water-Based Lubricant Additives. J. Am. Ceram. Soc. 2020, 103, 6951–6960. [Google Scholar] [CrossRef]
- Cheng, H.; Zhao, W. Regulating the Nb2C nanosheets with different degrees of oxidation in water lubricated sliding toward an excellent tribological performance. Friction 2021, 1–13. [Google Scholar] [CrossRef]
- Nguyen, H.; Chung, K. Assessment of Tribological Properties of Ti3C2 as a Water-Based Lubricant Additive. Materials 2020, 13, 5545. [Google Scholar] [CrossRef]
- Tang, W.; Jiang, Z.; Wang, B.; Li, Y. Black phosphorus quantum dots: A new-type of water-based high-efficiency lubricant additive. Friction 2021, 9, 1528–1542. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Chen, Z.; Wu, B.; Xu, S.; Luo, J. Layered Double Hydroxide Nanoplatelets with Excellent Tribological Properties under High Contact Pressure as Water-Based Lubricant Additives. Sci. Rep. 2016, 6, 22748. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Liu, W.; Liu, Y.; Wang, K.; Li, J.; Ma, T.; Eryilmaz, O.L.; Shi, Y.; Erdemir, A.; et al. Superlubricity of Polyalkylene Glycol Aqueous Solutions Enabled by Ultrathin Layered Double Hydroxide Nanosheets. ACS Appl. Mater. Interfaces 2019, 11, 20249–20256. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Z.; Dong, G. Preparation of nanoscale liquid metal droplet wrapped with chitosan and its tribological properties as water-based lubricant additive. Tribol. Int. 2020, 148, 106349. [Google Scholar] [CrossRef]
- Ye, X.; Wang, J.; Fan, M. Evaluating tribological properties of the stearic acid-based organic nanomaterials as additives for aqueous lubricants. Tribol. Int. 2019, 140, 105848. [Google Scholar] [CrossRef]
- Odzak, N.; Kistler, D.; Behra, R.; Sigg, L. Dissolution of metal and metal oxide nanoparticles in aqueous media. Environ. Pollut. 2014, 191, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Bang, I.C.; Onoe, J. Characteristic stability of bare Au-water nanofluids fabricated by pulsed laser ablation in liquids. Opt. Lasers Eng. 2009, 47, 532–538. [Google Scholar] [CrossRef]
- Lv, T.; Xu, X.; Yu, A.; Niu, C.; Hu, X. Ambient air quantity and cutting performances of water-based Fe3O4 nanofluid in magnetic minimum quantity lubrication. Int. J. Adv. Manuf. Technol. 2021, 115, 1711–1722. [Google Scholar] [CrossRef]
- Bao, Y.; Sun, J.; Kong, L. Effects of nano-SiO2 as water-based lubricant additive on surface qualities of strips after hot rolling. Tribol. Int. 2017, 114, 257–263. [Google Scholar] [CrossRef]
- Lv, T.; Xu, X.; Yu, A.; Hu, X. Oil mist concentration and machining characteristics of SiO2 water-based nano-lubricants in electrostatic minimum quantity lubrication-EMQL milling. J. Mater. Process. Technol. 2021, 290, 116964. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, G.; Oli, Y.; Zhang, Z.; Xue, Q. Study on tribological properties of oleic acid-modified TiO2 nanoparticle in water. Wear 2002, 252, 454–458. [Google Scholar] [CrossRef]
- Gu, Y.; Zhao, X.; Liu, Y.; Lv, Y. Preparation and Tribological Properties of Dual-Coated TiO2 Nanoparticles as Water-Based Lubricant Additives. J. Nanomater. 2014, 2014, 785680. [Google Scholar] [CrossRef] [Green Version]
- Ohenoja, K.; Saari, J.; Illikainen, M.; Niinimäki, J. Effect of molecular weight of sodium polyacrylates on the particle size distribution and stability of a TiO2 suspension in aqueous stirred media milling. Powder Technol. 2014, 262, 188–193. [Google Scholar] [CrossRef]
- Najiha, M.S.; Rahman, M.M. Experimental investigation of flank wear in end milling of aluminum alloy with water-based TiO2 nanofluid lubricant in minimum quantity lubrication technique. Int. J. Adv. Manuf. Technol. 2016, 86, 2527–2537. [Google Scholar] [CrossRef] [Green Version]
- Kayhani, M.H.; Soltanzadeh, H.; Heyhat, M.M.; Nazari, M.; Kowsary, F. Experimental study of convective heat transfer and pressure drop of TiO2/water nanofluid. Int. Commun. Heat Mass Transf. 2012, 39, 456–462. [Google Scholar] [CrossRef]
- Ukamanal, M.; Chandra Mishra, P.; Kumar Sahoo, A. Temperature distribution during AISI 316 steel turning under TiO2-water based nanofluid spray environments. Mater. Today Proc. 2018, 5, 20741–20749. [Google Scholar] [CrossRef]
- Wang, L.; Tieu, A.K.; Zhu, H.; Deng, G.; Cui, S.; Zhu, Q. A study of water-based lubricant with a mixture of polyphosphate and nano-TiO2 as additives for hot rolling process. Wear 2021, 477, 203895. [Google Scholar] [CrossRef]
- Cui, Y.; Ding, M.; Sui, T.; Zheng, W.; Qiao, G.; Yan, S.; Liu, X. Role of nanoparticle materials as water-based lubricant additives for ceramics. Tribol. Int. 2020, 142, 105978. [Google Scholar] [CrossRef]
- Wu, C.; Hou, S.X.; Zhang, H.Q.; Jia, X.M. Study and Evaluation on Dispersion of Molybdenum Disulfide in Aqueous Solution. Adv. Mater. Res. 2013, 750–752, 2175–2178. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, J. Tribological performances of multilayer-MoS2 nanoparticles in water-based lubricating fluid. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 182, p. 012023. [Google Scholar] [CrossRef]
- Xie, H.; Dang, S.; Jiang, B.; Xiang, L.; Zhou, S.; Sheng, H.; Yang, T.; Pan, F. Tribological performances of SiO2/graphene combinations as water-based lubricant additives for magnesium alloy rolling. Appl. Surf. Sci. 2019, 475, 847–856. [Google Scholar] [CrossRef]
- Xie, H.; Wei, Y.; Jiang, B.; Tang, C.; Nie, C. Tribological properties of carbon nanotube/SiO2 combinations as water-based lubricant additives for magnesium alloy. J. Mater. Res. Technol. 2021, 12, 138–149. [Google Scholar] [CrossRef]
- Zayan, M.; Rasheed, A.K.; John, A.; Khalid, M.; Ismail, A. Experimental Investigation on Rheological Properties of Water Based Novel Ternary Hybrid Nanofluids. Nanoscience 2021. [Google Scholar] [CrossRef]
- Wang, N.; Wang, H.; Ren, J.; Gao, G.; Chen, S.; Zhao, G.; Yang, Y.; Wang, J. Novel additive of PTFE@SiO2 core-shell nanoparticles with superior water lubricating properties. Mater. Des. 2020, 195, 109069. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Yu, L.; Zhang, Z.; Wu, Z.; Zhang, P. Preparation and tribological properties of water-soluble copper/silica nanocomposite as a water-based lubricant additive. Appl. Surf. Sci. 2012, 259, 824–830. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, C.; Gao, C.; Zhang, Y.; Yang, G.; Zhang, P.; Zhang, S. Preparation of Cu@SiO2 composite nanoparticle and its tribological properties as water-based lubricant additive. Lubr. Sci. 2020, 32, 69–79. [Google Scholar] [CrossRef]
- He, J.; Sun, J.; Meng, Y.; Pei, Y. Superior lubrication performance of MoS2-Al2O3 composite nanofluid in strips hot rolling. J. Manuf. Process. 2020, 57, 312–323. [Google Scholar] [CrossRef]
- Kumar, A.S.; Deb, S.; Paul, S. Tribological characteristics and micromilling performance of nanoparticle enhanced water based cutting fluids in minimum quantity lubrication. J. Manuf. Process. 2020, 56, 766–776. [Google Scholar] [CrossRef]
- Giwa, S.O.; Sharifpur, M.; Ahmadi, M.H.; Sohel Murshed, S.M.; Meyer, J.P. Experimental Investigation on Stability, Viscosity, and Electrical Conductivity of Water-Based Hybrid Nanofluid of MWCNT-Fe(2)O(3). Nanomaterials 2021, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Huang, J.; Jia, X.; Sheng, W. Facile synthesis of core–shell Ag@C nanospheres with improved tribological properties for water-based additives. New J. Chem. 2018, 42, 8773–8782. [Google Scholar] [CrossRef]
- Gara, L.; Zou, Q. Friction and Wear Characteristics of Water-Based ZnO and Al2O3 Nanofluids. Tribol. Trans. 2012, 55, 345–350. [Google Scholar] [CrossRef]
- Liang, D.; Ling, X.; Xiong, S. Preparation, characterisation and lubrication performances of Eu doped WO3 nanoparticle reinforce Mn3B7O13 Cl as water-based lubricant additive for laminated Cu-Fe composite sheet during hot rolling. Lubr. Sci. 2021, 33, 142–152. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, L.; Cheng, G.; Yuan, N.; Ding, J.; Pesika, N.S. Water-Based Lubrication of Hard Carbon Microspheres as Lubricating Additives. Tribol. Lett. 2018, 66, 148. [Google Scholar] [CrossRef]
- Peng, Y.; Hu, Y.; Wang, H. Tribological behaviors of surfactant-functionalized carbon nanotubes as lubricant additive in water. Tribol. Lett. 2007, 25, 247–253. [Google Scholar] [CrossRef]
- Sun, X.; Han, B.; Kang, H.; Fan, Z.; Liu, Y.; Umar, A.; Guo, Z. Frictional Reduction with Partially Exfoliated Multi-Walled Carbon Nanotubes as Water-Based Lubricant Additives. J. Nanosci. Nanotechnol. 2018, 18, 3427–3432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, C.; He, Z.; Liu, D.; Zhang, K.; Dong, C. Urea Modified Fluorinated Carbon Nanotubes: Unique Self-Dispersed Characteristic in Water and High Tribological Performance as Water-Based Lubricant Additives. New J. Chem. 2019, 43, 14684–14693. [Google Scholar] [CrossRef]
- Kristiansen, K.; Zeng, H.; Wang, P.; Israelachvili, J. Microtribology of Aqueous Carbon Nanotube Dispersions. Adv. Funct. Mater. 2011, 21, 4555–4564. [Google Scholar] [CrossRef]
- Ye, X.; E, S.; Fan, M. The influences of functionalized carbon nanotubes as lubricating additives: Length and diameter. Diam. Relat. Mater. 2019, 100, 107548. [Google Scholar] [CrossRef]
- Tang, W.; Wang, B.; Li, J.; Li, Y.; Zhang, Y.; Quan, H.; Huang, Z. Facile pyrolysis synthesis of ionic liquid capped carbon dots and subsequent application as the water-based lubricant additives. J. Mater. Sci. 2019, 54, 1171–1183. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, S.; Xu, Q.; Zhang, H. Carbon quantum dots: An innovative additive for water lubrication. Sci. China Technol. Sci. 2019, 62, 587–596. [Google Scholar] [CrossRef]
- Fan, K.; Liu, X.; Liu, Y.; Li, Y.; Chen, Y.; Meng, Y.; Liu, X.; Feng, W.; Luo, L. Covalent functionalization of fluorinated graphene through activation of dormant radicals for water-based lubricants. Carbon 2020, 167, 826–834. [Google Scholar] [CrossRef]
- Ma, L.; Li, Z.; Jia, W.; Hou, K.; Wang, J.; Yang, S. Microwave-assisted synthesis of hydroxyl modified fluorinated graphene with high fluorine content and its high load-bearing capacity as water lubricant additive for ceramic/steel contact. Colloids Surf. A 2021, 610, 125931. [Google Scholar] [CrossRef]
- Ye, X.; Ma, L.; Yang, Z.; Wang, J.; Wang, H.; Yang, S. Covalent Functionalization of Fluorinated Graphene and Subsequent Application as Water-based Lubricant Additive. ACS Appl. Mater. Interfaces 2016, 8, 7483–7488. [Google Scholar] [CrossRef] [PubMed]
- Qiang, R.; Hu, L.; Hou, K.; Wang, J.; Yang, S. Water-Soluble Graphene Quantum Dots as High-Performance Water-Based Lubricant Additive for Steel/Steel Contact. Tribol. Lett. 2019, 67, 64. [Google Scholar] [CrossRef]
- Piatkowska, A.; Romaniec, M.; Grzybek, D.; Mozdzonek, M.; Rojek, A.; Diduszko, R. A study on antiwear properties of graphene water-based lubricant and its contact with metallic materials. Tribologia 2018, 281, 71–81. [Google Scholar] [CrossRef]
- Mirzaamiri, R.; Akbarzadeh, S.; Ziaei-Rad, S.; Shin, D.-G.; Kim, D.-E. Molecular dynamics simulation and experimental investigation of tribological behavior of nanodiamonds in aqueous suspensions. Tribol. Int. 2021, 156, 106838. [Google Scholar] [CrossRef]
- Kinoshita, H.; Nishina, Y.; Alias, A.A.; Fujii, M. Tribological properties of monolayer graphene oxide sheets as water-based lubricant additives. Carbon 2014, 66, 720–723. [Google Scholar] [CrossRef]
- Elomaa, O.; Singh, V.K.; Iyer, A.; Hakala, T.J.; Koskinen, J. Graphene oxide in water lubrication on diamond-like carbon vs. stainless steel high-load contacts. Diam. Relat. Mater. 2015, 52, 43–48. [Google Scholar] [CrossRef]
- Singh, S.; Chen, X.; Zhang, C.; Tyagi, R.; Luo, J. Investigation on the lubrication potential of graphene oxide aqueous dispersion for self-mated stainless steel tribo-pair. Vacuum 2019, 166, 307–315. [Google Scholar] [CrossRef]
- Bai, M.; Liu, J.; He, J.; Li, W.; Wei, J.; Chen, L.; Miao, J.; Li, C. Heat transfer and mechanical friction reduction properties of graphene oxide nanofluids. Diam. Relat. Mater. 2020, 108, 107982. [Google Scholar] [CrossRef]
- Gan, C.; Liang, T.; Li, X.; Li, W.; Li, H.; Fan, X.; Zhu, M. Ultra-dispersive monolayer graphene oxide as water-based lubricant additive: Preparation, characterization and lubricating mechanisms. Tribol. Int. 2021, 155, 106768. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, D.-E. Water Lubrication of Stainless Steel using Reduced Graphene Oxide Coating. Sci. Rep. 2015, 5, 17034. [Google Scholar] [CrossRef]
- Kinoshita, H.; Suzuki, K.; Suzuki, T.; Nishina, Y. Tribological properties of oxidized wood-derived nanocarbons with same surface chemical composition as graphene oxide for additives in water-based lubricants. Diam. Relat. Mater. 2018, 90, 101–108. [Google Scholar] [CrossRef]
- Wei, Q.; Fu, T.; Yue, Q.; Liu, H.; Ma, S.; Cai, M.; Zhou, F. Graphene oxide/brush-like polysaccharide copolymer nanohybrids as eco-friendly additives for water-based lubrication. Tribol. Int. 2021, 157, 106895. [Google Scholar] [CrossRef]
- Li, X.; Lu, H.; Guo, J.; Tong, Z.; Dong, G. Synergistic water lubrication effect of self-assembled nanofilm and graphene oxide additive. Appl. Surf. Sci. 2018, 455, 1070–1077. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Zeng, Z.; Zhao, H.; Ge, X.; Wang, K.; Wang, L.; Xue, Q. PEGlated graphene as nanoadditive for enhancing the tribological properties of water-based lubricants. Carbon 2018, 137, 41–48. [Google Scholar] [CrossRef]
- Shariatzadeh, M.; Grecov, D. Cellulose Nanocrystals Suspensions as Water-Based Lubricants for Slurry Pump Gland Seals. Int. J. Aerosp. Mech. Eng. 2018, 12, 603–607. [Google Scholar] [CrossRef]
- Shariatzadeh, M.; Grecov, D. Aqueous suspensions of cellulose nanocrystals as water-based lubricants. Cellulose 2019, 26, 4665–4677. [Google Scholar] [CrossRef]
- Lei, H.; Guan, W.; Luo, J. Tribological behavior of fullerene–styrene sulfonic acid copolymer as water-based lubricant additive. Wear 2002, 252, 345–350. [Google Scholar] [CrossRef]
- Jiang, G.; Guan, W.; Zheng, Q. A study on fullerene–acrylamide copolymer nanoball—A new type of water-based lubrication additive. Wear 2005, 258, 1625–1629. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, W.; Yu, Q.; Zhang, J.; Ma, Z.; Zhang, M.; Zhang, L.; Bai, Y.; Cai, M.; Zhou, F.; et al. Significantly Reducing Friction and Wear of Water-Based Fluids with Shear Thinning Bicomponent Supramolecular Hydrogels. Adv. Mater. Interfaces 2020, 7, 2001084. [Google Scholar] [CrossRef]
- Yang, D.; Du, X.; Li, W.; Han, Y.; Ma, L.; Fan, M.; Zhou, F.; Liu, W. Facile Preparation and Tribological Properties of Water-Based Naphthalene Dicarboxylate Ionic Liquid Lubricating Additives. Tribol. Lett. 2020, 68, 84. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Zhang, S.; Yu, L.; Zhang, P.; Zhang, Z. Preparation of Water-Soluble Lanthanum Fluoride Nanoparticles and Evaluation of their Tribological Properties. Tribol. Lett. 2013, 52, 305–314. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, X.; Liu, Z.; Ju, C.; Xu, Z.; Xu, J.; Yang, C. Synergy between two protic ionic liquids for improving the antiwear property of glycerol aqueous solution. Tribol. Int. 2020, 141, 105731. [Google Scholar] [CrossRef]
- Dong, R.; Yu, Q.; Bai, Y.; Wu, Y.; Ma, Z.; Zhang, J.; Zhang, C.; Yu, B.; Zhou, F.; Liu, W.; et al. Towards superior lubricity and anticorrosion performances of proton-type ionic liquids additives for water-based lubricating fluids. Chem. Eng. J. 2020, 383, 123201. [Google Scholar] [CrossRef]
- Khanmohammadi, H.; Wijanarko, W.; Espallargas, N. Ionic Liquids as Additives in Water-Based Lubricants: From Surface Adsorption to Tribofilm Formation. Tribol. Lett. 2020, 68, 130. [Google Scholar] [CrossRef]
- Kreivaitis, R.; Gumbytė, M.; Kupčinskas, A.; Kazancev, K.; Ta, T.N.; Horng, J.H. Investigation of tribological properties of two protic ionic liquids as additives in water for steel–steel and alumina–steel contacts. Wear 2020, 456–457, 203390. [Google Scholar] [CrossRef]
- He, J.; Sun, J.; Meng, Y.; Yan, X. Preliminary investigations on the tribological performance of hexagonal boron nitride nanofluids as lubricant for steel/steel friction pairs. Surf. Topogr. Metrol. Prop. 2019, 7, 015022. [Google Scholar] [CrossRef]
- Abdollah, M.F.B.; Amiruddin, H.; Azmi, M.A.; Tahir, N.A.M. Lubrication mechanisms of hexagonal boron nitride nano-additives water-based lubricant for steel–steel contact. J. Eng. Tribol. 2020, 235, 1038–1046. [Google Scholar] [CrossRef]
- Lin, B.; Ding, M.; Sui, T.; Cui, Y.; Yan, S.; Liu, X. Excellent Water Lubrication Additives for Silicon Nitride to Achieve Superlubricity under Extreme Conditions. Langmuir 2019, 35, 14861–14869. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xie, G.; Luo, J. Black phosphorus as a new lubricant. Friction 2018, 6, 116–142. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Hou, T.; Wang, W.; Zhang, G.; Gao, Y.; Wang, K. Tribological behavior of black phosphorus nanosheets as water-based lubrication additives. Friction 2021, 1–14. [Google Scholar] [CrossRef]
- Ali, N.; Amaral Teixeira, J.; Addali, A. A Review on Nanofluids: Fabrication, Stability, and Thermophysical Properties. J. Nanomater. 2018, 2018, 6978130. [Google Scholar] [CrossRef]
- Dey, D.; Kumar, P.; Samantaray, S. A review of nanofluid preparation, stability, and thermo-physical properties. Heat Transf. Asian Res. 2017, 46, 1413–1442. [Google Scholar] [CrossRef]
- Ghadimi, A.; Saidur, R.; Metselaar, H.S.C. A review of nanofluid stability properties and characterization in stationary conditions. Int. J. Heat Mass Transf. 2011, 54, 4051–4068. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, H.; Wang, L.; Liu, L. Critical Issues in Nanofluids Preparation, Characterization and Thermal Conductivity. Curr. Nanosci. 2009, 5, 103–112. [Google Scholar] [CrossRef]
- Zhu, H.; Li, C.; Wu, D.; Zhang, C.; Yin, Y. Preparation, characterization, viscosity and thermal conductivity of CaCO3 aqueous nanofluids. Sci. China Technol. Sci. 2010, 53, 360–368. [Google Scholar] [CrossRef]
- Urmi, W.T.; Rahman, M.M.; Kadirgama, K.; Ramasamy, D.; Maleque, M.A. An overview on synthesis, stability, opportunities and challenges of nanofluids. Mater. Today Proc. 2021, 41, 30–37. [Google Scholar] [CrossRef]
- Fang, Y.; Ma, L.; Luo, J. Modelling for water-based liquid lubrication with ultra-low friction coefficient in rough surface point contact. Tribol. Int. 2020, 141, 105901. [Google Scholar] [CrossRef]
- Azman, N.F.; Samion, S. Dispersion Stability and Lubrication Mechanism of Nanolubricants: A Review. Int. J. Precis. Eng. Manuf.-Green Technol. 2019, 6, 393–414. [Google Scholar] [CrossRef]
- Cacua, K.; Murshed, S.M.S.; Pabón, E.; Buitrago, R. Dispersion and thermal conductivity of TiO2/water nanofluid: Effects of ultrasonication, agitation and temperature. J. Therm. Anal. Calorim. 2019, 140, 109–114. [Google Scholar] [CrossRef]
- Lee, K.; Hwang, Y.; Cheong, S.; Kwon, L.; Kim, S.; Lee, J. Performance evaluation of nano-lubricants of fullerene nanoparticles in refrigeration mineral oil. Curr. Appl. Phys. 2009, 9, e128–e131. [Google Scholar] [CrossRef]
- Ghadimi, A.; Metselaar, H.; LotfizadehDehkordi, B. Nanofluid stability optimization based on UV-Vis spectrophotometer measurement. J. Eng. Sci. Technol. 2015, 10, 32–40. [Google Scholar]
- Jiang, L.; Gao, L.; Sun, J. Production of aqueous colloidal dispersions of carbon nanotubes. J. Colloid Interface Sci. 2003, 260, 89–94. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H. A Review on Nanofluids: Preparation, Stability Mechanisms, and Applications. J. Nanomater. 2012, 2012, 128. [Google Scholar] [CrossRef] [Green Version]
- Sajid, M.U.; Ali, H.M. Thermal conductivity of hybrid nanofluids: A critical review. Int. J. Heat Mass Transf. 2018, 126, 211–234. [Google Scholar] [CrossRef]
- Alawi, O.A.; Mallah, A.R.; Kazi, S.N.; Sidik, N.A.C.; Najafi, G. Thermophysical properties and stability of carbon nanostructures and metallic oxides nanofluids. J. Therm. Anal. Calorim. 2019, 135, 1545–1562. [Google Scholar] [CrossRef]
- Kumar, M.S.; Vasu, V.; Gopal, A.V. Thermal conductivity and rheological studies for Cu–Zn hybrid nanofluids with various basefluids. J. Taiwan Inst. Chem. Eng. 2016, 66, 321–327. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Y.; Liang, X.; Xu, J.; Lee, C.; Liang, Q.; Tao, P.; Deng, T. Dispersion stability of thermal nanofluids. Prog. Nat. Sci. Mater. Int. 2017, 27, 531–542. [Google Scholar] [CrossRef]
- Cremaschi, L.; Bigi, A.; Wong, T.; Deokar, P. Thermodynamic properties of Al2O3 nanolubricants: Part 1—Effects on the two-phase pressure drop. Sci. Technol. Built Environ. 2015, 21, 607–620. [Google Scholar] [CrossRef]
- Huminic, G.; Huminic, A.; Fleacă, C.; Dumitrache, F.; Morjan, I. Experimental study on viscosity of water based Fe–Si hybrid nanofluids. J. Mol. Liq. 2021, 321, 114938. [Google Scholar] [CrossRef]
- Haghighi, E.; Nikkam, N.; Saleemi, M.; Behi, R.; Mirmohammadi, S.A.; Poth, H.; Khodabandeh, R.; Toprak, M.; Muhammed, M.; Palm, B. Shelf stability of nanofluids and its effect on thermal conductivity and viscosity Shelf stability of nanofluids and its effect on thermal conductivity and viscosity. Meas. Sci. Technol. 2013, 24, 105301–105311. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Rashidi, A.; Soleimanisalim, A.H.; Rashtchian, D. Investigating the rheological properties of nanofluids of water/hybrid nanostructure of spherical silica/MWCNT. Thermochim. Acta 2014, 578, 53–58. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, C.; Tang, Y.; Wang, J.; Ren, B.; Yin, Y. Preparation and thermal conductivity of suspensions of graphite nanoparticles. Carbon 2007, 45, 226–228. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Tung, S.; Schneider, E.; Xi, S. A review on development of nanofluid preparation and characterization. Powder Technol. 2009, 196, 89–101. [Google Scholar] [CrossRef]
- Mukherjee, S.; Paria, S. Preparation and Stability of Nanofluids-A Review. IOSR J. Mech. Civ. Eng. 2013, 9, 63–69. [Google Scholar] [CrossRef]
- Qamar, A.; Anwar, Z.; Ali, H.; Shaukat, R.; Imran, S.; Arshad, A.; Ali, H.M.; Korakianitis, T. Preparation and dispersion stability of aqueous metal oxide nanofluids for potential heat transfer applications: A review of experimental studies. J. Therm. Anal. Calorim. 2020, 1–24. [Google Scholar] [CrossRef]
- Oh, D.-W.; Jain, A.; Eaton, J.K.; Goodson, K.E.; Lee, J.S. Thermal conductivity measurement and sedimentation detection of aluminum oxide nanofluids by using the 3ω method. Int. J. Heat Fluid Flow 2008, 29, 1456–1461. [Google Scholar] [CrossRef]
- Peng, X.F.; Yu, X.; Xia, L.F.; Zhong, X. Influence factors on suspension stability of nanofluids. J. Zhejiang Univ. 2007, 41, 577–580. [Google Scholar]
- Azizian, R.; Doroodchi, E.; Moghtaderi, B. Influence of Controlled Aggregation on Thermal Conductivity of Nanofluids. J. Heat Transf. 2016, 138, 021301. [Google Scholar] [CrossRef] [Green Version]
- Jama, M.; Singh, T.; Mahmoud, S.; Koç, M.; Samara, A.; Isaifan, R.; Atieh, M. Critical Review on Nanofluids: Preparation, Characterization, and Applications. J. Nanomater. 2016, 2016, 6717624. [Google Scholar] [CrossRef] [Green Version]
- Kumar Dubey, M.; Bijwe, J.; Ramakumar, S.S.V. PTFE based nano-lubricants. Wear 2013, 306, 80–88. [Google Scholar] [CrossRef]
- Lee, G.-J.; Rhee, C.K. Enhanced thermal conductivity of nanofluids containing graphene nanoplatelets prepared by ultrasound irradiation. J. Mater. Sci. 2014, 49, 1506–1511. [Google Scholar] [CrossRef]
- Mahbubul, I.M.; Elcioglu, E.B.; Saidur, R.; Amalina, M.A. Optimization of ultrasonication period for better dispersion and stability of TiO2–water nanofluid. Ultrason. Sonochem. 2017, 37, 360–367. [Google Scholar] [CrossRef]
- Tang, E.; Cheng, G.; Ma, X.; Pang, X.; Zhao, Q. Surface modification of zinc oxide nanoparticle by PMAA and its dispersion in aqueous system. Appl. Surf. Sci. 2006, 252, 5227–5232. [Google Scholar] [CrossRef]
- Neouze, M.-A.; Schubert, U. Surface Modification and Functionalization of Metal and Metal Oxide Nanoparticles by Organic Ligands. Mon. Chem.-Chem. Mon. 2008, 139, 183–195. [Google Scholar] [CrossRef]
- Yang, X.-F.; Liu, Z.-H. Pool boiling heat transfer of functionalized nanofluid under sub-atmospheric pressures. Int. J. Therm. Sci. 2011, 50, 2402–2412. [Google Scholar] [CrossRef]
- Sezer, N.; Atieh, M.A.; Koç, M. A comprehensive review on synthesis, stability, thermophysical properties, and characterization of nanofluids. Powder Technol. 2019, 344, 404–431. [Google Scholar] [CrossRef]
- Narendar, G.; Gupta, A.V.S.S.K.S.; Krishnaiah, A.; Satyanarayana, M.G.V. Experimental investigation on the preparation and applications of Nano fluids. Mater. Today Proc. 2017, 4, 3926–3931. [Google Scholar] [CrossRef]
- Xia, G.; Jiang, H.; Liu, R.; Zhai, Y. Effects of surfactant on the stability and thermal conductivity of Al2O3/de-ionized water nanofluids. Int. J. Therm. Sci. 2014, 84, 118–124. [Google Scholar] [CrossRef]
- Kakati, H.; Mandal, A.; Laik, S. Promoting effect of Al2O3/ZnO-based nanofluids stabilized by SDS surfactant on CH4+C2H6+C3H8 hydrate formation. J. Ind. Eng. Chem. 2016, 35, 357–368. [Google Scholar] [CrossRef]
- Tomala, A.; Karpinska, A.; Werner, W.S.M.; Olver, A.; Störi, H. Tribological properties of additives for water-based lubricants. Wear 2010, 269, 804–810. [Google Scholar] [CrossRef]
- Wen, P.; Lei, Y.; Li, W.; Fan, M. Synergy between Covalent Organic Frameworks and Surfactants to Promote Water-Based Lubrication and Corrosion-Resistance. ACS Appl. Nano Mater. 2020, 3, 1400–1411. [Google Scholar] [CrossRef]
- Popa, I.; Gillies, G.; Papastavrou, G.; Borkovec, M. Attractive and Repulsive Electrostatic Forces between Positively Charged Latex Particles in the Presence of Anionic Linear Polyelectrolytes. J. Phys. Chem. B 2010, 114, 3170–3177. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T. Chapter 2—Colloid and interface aspects of pharmaceutical science. In Colloid and Interface Science in Pharmaceutical Research and Development; Ohshima, H., Makino, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 29–54. [Google Scholar]
- Du, Y.; Yuan, X. Coupled hybrid nanoparticles for improved dispersion stability of nanosuspensions: A review. J. Nanopart. Res. 2020, 22, 261. [Google Scholar] [CrossRef]
- Byrd, T.; Walz, J. Interaction Force Profiles between Cryptosporidium parvum Oocysts and Silica Surfaces. Environ. Sci. Technol. 2006, 39, 9574–9582. [Google Scholar] [CrossRef] [PubMed]
- Napper, D.H. Steric stabilization. J. Colloid Interface Sci. 1977, 58, 390–407. [Google Scholar] [CrossRef]
- Zhulina, E.B.; Borisov, O.V.; Priamitsyn, V.A. Theory of steric stabilization of colloid dispersions by grafted polymers. J. Colloid Interface Sci. 1990, 137, 495–511. [Google Scholar] [CrossRef]
- Gerber, P.; Moore, M.A. Comments on the Theory of Steric Stabilization. Macromolecules 1977, 10, 476–481. [Google Scholar] [CrossRef]
- Dutta, N.; Green, D. Impact of Solvent Quality on Nanoparticle Dispersion in Semidilute and Concentrated Polymer Solutions. Langmuir 2010, 26, 16737–16744. [Google Scholar] [CrossRef]
- Munkhbayar, B.; Tanshen, M.R.; Jeoun, J.; Chung, H.; Jeong, H. Surfactant-free dispersion of silver nanoparticles into MWCNT-aqueous nanofluids prepared by one-step technique and their thermal characteristics. Ceram. Int. 2013, 39, 6415–6425. [Google Scholar] [CrossRef]
- Hatami, M.; Ali, F.; Alsabery, A.; Hu, S.; Jing, D.; Hameed, K. mixed convection heat transfer of SiO2-water and alumina-pao nano-lubricants used in a mechanical ball bearing. J. Therm. Eng. 2021, 7, 134–161. [Google Scholar] [CrossRef]
- Chen, L.; Cheng, M.; Yang, D.; Yang, L. Enhanced Thermal Conductivity of Nanofluid by Synergistic Effect of Multi-Walled Carbon Nanotubes and Fe2O3 Nanoparticles. Appl. Mech. Mater. 2014, 548–549, 118–123. [Google Scholar] [CrossRef]
- Botha, S.S.; Ndungu, P.; Bladergroen, B.J. Physicochemical Properties of Oil-Based Nanofluids Containing Hybrid Structures of Silver Nanoparticles Supported on Silica. Ind. Eng. Chem. Res. 2011, 50, 3071–3077. [Google Scholar] [CrossRef]
- Nine, M.J.; Munkhbayar, B.; Rahman, M.S.; Chung, H.; Jeong, H. Highly productive synthesis process of well dispersed Cu2O and Cu/Cu2O nanoparticles and its thermal characterization. Mater. Chem. Phys. 2013, 141, 636–642. [Google Scholar] [CrossRef]
- Valdes, M.; Gonzalo, J.; Marín, A.; Rodriguez, M.; Betancur, J. Tribometry: How is friction research quantified? A review. Int. J. Eng. Res. Technol. 2020, 13, 2596–2610. [Google Scholar] [CrossRef]
- Paul, G.; Hirani, H.; Kuila, T.; Murmu, N. Nanolubricants Dispersed with Graphene and its Derivatives: An Assessment and Review of the Tribological Performance. Nanoscale 2019, 11, 3458–3483. [Google Scholar] [CrossRef] [PubMed]
- Sagraloff, N.; Dobler, A.; Tobie, T.; Stahl, K.; Ostrowski, J. Development of an Oil Free Water-Based Lubricant for Gear Applications. Lubricants 2019, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Jiang, F.; Yan, L.; Jiang, Z.; Xu, X. A novel ultrahigh-speed ball-on-disc tribometer. Tribol. Int. 2021, 157, 106901. [Google Scholar] [CrossRef]
- Chen, W.; Amann, T.; Kailer, A.; Rühe, J. Macroscopic Friction Studies of Alkylglucopyranosides as Additives for Water-Based Lubricants. Lubricants 2020, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Jiang, Z.; Wei, D.; Wu, H.; Jiang, L. Adhesion, friction and wear analysis of a chromium oxide scale on a ferritic stainless steel. Wear 2019, 426–427, 1212–1221. [Google Scholar] [CrossRef]
- Pawlak, Z.; Urbaniak, W.; Oloyede, A. The relationship between friction and wettability in aqueous environment. Wear 2011, 271, 1745–1749. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Li, J.; Luo, J. Enhancement of friction performance enabled by a synergetic effect between graphene oxide and molybdenum disulfide. Carbon 2019, 154, 266–276. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Lu, Y.; Li, Z.; Cheng, X.; Hasan, M.; Zhang, H.; Jiang, Z. Influences of Load and Microstructure on Tribocorrosion Behaviour of High Strength Hull Steel in Saline Solution. Tribol. Lett. 2019, 67, 124. [Google Scholar] [CrossRef]
- Tang, W.; Zhu, X.; Li, Y. Tribological performance of various metal-doped carbon dots as water-based lubricant additives and their potential application as additives of poly(ethylene glycol). Friction 2021, 1, 1–18. [Google Scholar] [CrossRef]
- Kim, H.J.; Shin, D.G.; Kim, D.-E. Frictional behavior between silicon and steel coated with graphene oxide in dry sliding and water lubrication conditions. Int. J. Precis. Eng. Manuf.-Green Technol. 2016, 3, 91–97. [Google Scholar] [CrossRef]
- Rosa, W.O.; Vereda, F.; de Vicente, J. Tribological Behavior of Glycerol/Water-Based Magnetorheological Fluids in PMMA Point Contacts. Front. Mater. 2019, 6, 32. [Google Scholar] [CrossRef] [Green Version]
- Kotia, A.; Borkakoti, S.; Deval, P.; Ghosh, S.K. Review of interfacial layer’s effect on thermal conductivity in nanofluid. Heat Mass Transf. 2017, 53, 2199–2209. [Google Scholar] [CrossRef]
- Kotia, A.; Rajkhowa, P.; Rao, G.S.; Ghosh, S.K. Thermophysical and tribological properties of nanolubricants: A review. Heat Mass Transf. 2018, 54, 3493–3508. [Google Scholar] [CrossRef]
- Tang, Z.; Li, S. A review of recent developments of friction modifiers for liquid lubricants (2007–present). Curr. Opin. Solid State Mater. Sci. 2014, 18, 119–139. [Google Scholar] [CrossRef]
- Kong, L.; Sun, J.; Bao, Y. Preparation, characterization and tribological mechanism of nanofluids. RSC Adv. 2017, 7, 12599–12609. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhou, M.; Jin, L.; Li, L.; Mo, Y.; Su, G.; Li, X.; Zhu, H.; Tian, Y. Recent advances in friction and lubrication of graphene and other 2D materials: Mechanisms and applications. Friction 2019, 7, 199–216. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Huang, Y.; He, Y.; Shi, Y. Nanolubricant additives: A review. Friction 2021, 9, 891–917. [Google Scholar] [CrossRef]
- Sarno, M.; Scarpa, D.; Senatore, A.; Mustafa, W. rGO/GO Nanosheets in Tribology: From the State of the Art to the Future Prospective. Lubricants 2020, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Laad, M.; Jatti, V.K.S. Titanium oxide nanoparticles as additives in engine oil. J. King Saud Univ. Eng. Sci. 2018, 30, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Khadem, M.; Penkov, O.; Pukha, V.; Maleyev, M.; Kim, D.-E. Ultra-thin carbon-based nanocomposite coatings for superior wear resistance under lubrication with nano-diamond additives. RSC Adv. 2016, 6, 56918–56929. [Google Scholar] [CrossRef]
- Chiñas-Castillo, F.; Spikes, H. Mechanism of Action of Colloidal Solid Dispersions. J. Tribol. 2003, 125, 552–557. [Google Scholar] [CrossRef]
- Rapoport, L.; Leshchynsky, V.; Lvovsky, M.; Nepomnyashchy, O.; Volovik, Y.; Tenne, R. Mechanism of friction of fullerene. Ind. Lubr. Tribol. 2002, 54, 171–176. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Tsui, W.C.; Liu, T.C. Experimental analysis of tribological properties of lubricating oils with nanoparticle additives. Wear 2007, 262, 819–825. [Google Scholar] [CrossRef]
- Aldana, P.U.; Dassenoy, F.; Vacher, B.; Le Mogne, T.; Thiebaut, B. WS2 nanoparticles anti-wear and friction reducing properties on rough surfaces in the presence of ZDDP additive. Tribol. Int. 2016, 102, 213–221. [Google Scholar] [CrossRef]
- Ku, B.-C.; Han, Y.-C.; Lee, J.-E.; Lee, J.-K.; Park, S.-H.; Hwang, Y.-J. Tribological effects of fullerene (C60) nanoparticles added in mineral lubricants according to its viscosity. Int. J. Precis. Eng. Manuf. 2010, 11, 607–611. [Google Scholar] [CrossRef]
- Peng, D.; Kang, Y.; Hwang, R.; Shyr, S.; Chang, Y. Tribological properties of diamond and SiO2 nanoparticles added in paraffin. Tribol. Int. 2009, 42, 911–917. [Google Scholar] [CrossRef]
- Srivyas, P.; Charoo, M.S. A Review on Tribological Characterization of Lubricants with Nano Additives for Automotive Applications. Tribol. Ind. 2018, 40, 594–623. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, S. 2D nanomaterials as lubricant additive: A review. Mater. Des. 2017, 135, 319–332. [Google Scholar] [CrossRef]
- Flores-Castañeda, M.; Camps, E.; Camacho-López, M.; Muhl, S.; García, E.; Figueroa, M. Bismuth nanoparticles synthesized by laser ablation in lubricant oils for tribological tests. J. Alloy Compd. 2015, 643, S67–S70. [Google Scholar] [CrossRef]
- Kato, H.; Komai, K. Tribofilm formation and mild wear by tribo-sintering of nanometer-sized oxide particles on rubbing steel surfaces. Wear 2007, 262, 36–41. [Google Scholar] [CrossRef]
- Song, X.; Zheng, S.; Zhang, J.; Li, W.; Chen, Q.; Cao, B. Synthesis of monodispersed ZnAl2O4 nanoparticles and their tribology properties as lubricant additives. Mater. Res. Bull. 2012, 47, 4305–4310. [Google Scholar] [CrossRef]
- Uflyand, I.E.; Zhinzhilo, V.A.; Burlakova, V.E. Metal-containing nanomaterials as lubricant additives: State-of-the-art and future development. Friction 2019, 7, 93–116. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-G.; Hwang, Y.-J.; Choi, Y.-M.; Lee, J.-K.; Choi, C.; Oh, J.-M. A study on the tribological characteristics of graphite nano lubricants. Int. J. Precis. Eng. Manuf. 2009, 10, 85–90. [Google Scholar] [CrossRef]
- Lee, K.; Hwang, Y.; Cheong, S.; Choi, Y.; Kwon, L.; Lee, J.; Kim, S.H. Understanding the Role of Nanoparticles in Nano-oil Lubrication. Tribol. Lett. 2009, 35, 127–131. [Google Scholar] [CrossRef]
- Tevet, O.; Von-Huth, P.; Popovitz-Biro, R.; Rosentsveig, R.; Wagner, H.D.; Tenne, R. Friction mechanism of individual multilayered nanoparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 19901–19906. [Google Scholar] [CrossRef] [Green Version]
- Cizaire, L.; Vacher, B.; Le Mogne, T.; Martin, J.M.; Rapoport, L.; Margolin, A.; Tenne, R. Mechanisms of ultra-low friction by hollow inorganic fullerene-like MoS2 nanoparticles. Surf. Coat. Technol. 2002, 160, 282–287. [Google Scholar] [CrossRef]
- Joly-Pottuz, L.; Martin, J.; Dassenoy, F.; Belin, M.; Montagnac, G.; Reynard, B.; Fleischer, N. Pressure-induced exfoliation of inorganic fullerene-like WS2 particles in a Hertzian contact. J. Appl. Phys. 2006, 99, 023524. [Google Scholar] [CrossRef]
- Rapoport, L.; Feldman, Y.; Homyonfer, M.; Cohen, H.; Sloan, J.; Hutchison, J.L.; Tenne, R. Inorganic fullerene-like material as additives to lubricants: Structure–function relationship. Wear 1999, 225–229, 975–982. [Google Scholar] [CrossRef]
- Spikes, H. Friction Modifier Additives. Tribol. Lett. 2015, 60, 5. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Klein, J. Control of surface forces through hydrated boundary layers. Curr. Opin. Colloid Interface Sci. 2019, 44, 94–106. [Google Scholar] [CrossRef]
- Klein, J. Hydration lubrication. Friction 2013, 1, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Suresh, S.; Venkitaraj, K.P.; Selvakumar, P.; Chandrasekar, M. Synthesis of Al2O3–Cu/water hybrid nanofluids using two step method and its thermo physical properties. Colloids Surf. A 2011, 388, 41–48. [Google Scholar] [CrossRef]
- Kedzierski, M.A.; Brignoli, R.; Quine, K.T.; Brown, J.S. Viscosity, density, and thermal conductivity of aluminum oxide and zinc oxide nanolubricants. Int. J. Refrig. 2017, 74, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Mehrali, M.; Sadeghinezhad, E.; Latibari, S.T.; Kazi, S.N.; Mehrali, M.; Zubir, M.N.B.M.; Metselaar, H.S.C. Investigation of thermal conductivity and rheological properties of nanofluids containing graphene nanoplatelets. Nanoscale Res. Lett. 2014, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, D.P.; Das, D.K.; Chukwu, G.A. Temperature dependent rheological property of copper oxide nanoparticles suspension (nanofluid). J. Nanosci. Nanotechnol. 2006, 6, 1150–1154. [Google Scholar] [CrossRef]
- Pawelski, O.; Rasp, W.; Draese, S. Influence of Hydrodynamic Lubricant Entrainment on Friction Effects in Cold-Rolling. Steel Res. 1994, 65, 488–493. [Google Scholar] [CrossRef]
- Qu, J.; Truhan, J.J.; Dai, S.; Luo, H.; Blau, P.J. Ionic liquids with ammonium cations as lubricants or additives. Tribol. Lett. 2006, 22, 207–214. [Google Scholar] [CrossRef]
- Hild, W.; Opitz, A.; Schaefer, J.A.; Scherge, M. The effect of wetting on the microhydrodynamics of surfaces lubricated with water and oil. Wear 2003, 254, 871–875. [Google Scholar] [CrossRef]
- Xia, W.Z.; Zhao, J.W.; Wu, H.; Zhao, X.M.; Zhang, X.M.; Xu, J.Z.; Hee, A.C.; Jiang, Z.Y. Effects of Nano-TiO2 Additive in Oil-in-Water Lubricant on Contact Angle and Antiscratch Behavior. Tribol. Trans. 2017, 60, 362–372. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Tang, J.; Sun, W.; Tieu, A.K.; Wei, D. Analysis of tribological feature of the oxide scale in hot strip rolling. Tribol. Int. 2010, 43, 1339–1345. [Google Scholar] [CrossRef]
- Hao, L.; Wu, H.; Wei, D.B.; Cheng, X.W.; Zhao, J.W.; Luo, S.Z.; Jiang, L.Z.; Jiang, Z.Y. Wear and friction behaviour of high-speed steel and indefinite chill material for rolling ferritic stainless steels. Wear 2017, 376, 1580–1585. [Google Scholar] [CrossRef] [Green Version]
- Colás, R.; Ramírez, J.; Sandoval, I.; Morales, J.C.; Leduc, L.A. Damage in hot rolling work rolls. Wear 1999, 230, 56–60. [Google Scholar] [CrossRef]
- Najiha, M.S.; Rahman, M.M.; Kadirgama, K. Performance of water-based TiO2 nanofluid during the minimum quantity lubrication machining of aluminium alloy, AA6061-T6. J. Clean. Prod. 2016, 135, 1623–1636. [Google Scholar] [CrossRef] [Green Version]
- Williams, K. Tribology in Metal Working±New Developments. In Proceedings of the Echanical Engineering Conference, London, UK, 7–9 May 1980; pp. 4–6. [Google Scholar]
- Zhu, Z.; Sun, J.; Niu, T.; Liu, N. Experimental research on tribological performance of water-based rolling liquid containing nano-TiO2. J. Nanomater. Nanoeng. Nanosyst. 2015, 229, 104–109. [Google Scholar] [CrossRef]
- Jia, T.; Liu, Z.Y.; Hu, H.F.; Wang, G.D. The Optimal Design for the Production of Hot Rolled Strip with “Tight Oxide Scale” by Using Multi-objective Optimization. ISIJ Int. 2011, 51, 1468–1473. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.Y.; Tieu, A.K.; Sun, W.H.; Tang, J.N.; Wei, D.B. Characterisation of thin oxide scale and its surface roughness in hot metal rolling. Mater. Sci. Eng. A 2006, 435–436, 434–438. [Google Scholar] [CrossRef]
- Xiong, S.; Liang, D.; Wu, H.; Lin, W.; Chen, J.; Zhang, B. Preparation, characterization, tribological and lubrication performances of Eu doped CaWO4 nanoparticle as anti-wear additive in water-soluble fluid for steel strip during hot rolling. Appl. Surf. Sci. 2021, 539, 148090. [Google Scholar] [CrossRef]
- Cheng, X.; Jiang, Z.; Wei, D.; Hao, L.; Zhao, J.; Jiang, L. Oxide scale characterization of ferritic stainless steel and its deformation and friction in hot rolling. Tribol. Int. 2015, 84, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Jiang, Z.; Zhao, J.; Wei, D.; Zhou, C.; Huang, Q. Microstructure and microtexture evolutions of deformed oxide layers on a hot-rolled microalloyed steel. Corros. Sci. 2015, 90, 140–152. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, Z.; Zhao, J.; Wei, D.; Zhou, C.; Huang, Q. Effects of grain boundaries in oxide scale on tribological properties of nanoparticles lubrication. Wear 2015, 332–333, 1286–1292. [Google Scholar] [CrossRef] [Green Version]
- Dohda, K.; Boher, C.; Rezai-Aria, F.; Mahayotsanun, N. Tribology in metal forming at elevated temperatures. Friction 2015, 3, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Tominaga, J.; Wakimoto, K.; Mori, T.; Murakami, M.; Yoshimura, T. Manufacture of wire rods with good descaling property. Trans. ISIJ 1982, 22, 646–656. [Google Scholar] [CrossRef]
- Sun, W.; Tieu, A.K.; Jiang, Z.; Zhu, H.; Lu, C. Oxide scales growth of low-carbon steel at high temperatures. J. Mater. Process. Technol. 2004, 155–156, 1300–1306. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, Z. Thermomechanical processing of advanced high strength steels. Prog. Mater. Sci. 2018, 94, 174–242. [Google Scholar] [CrossRef]
- Verlinden, B.; Driver, J.; Samajdar, I.; Doherty, R.D. Thermo-Mechanical Processing of Metallic Materials; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Hou, H.; Chen, Q.; Liu, Q.; Dong, H. Grain refinement of a Nb–Ti microalloyed steel through heavy deformation controlled cooling. J. Mater. Process. Technol. 2003, 137, 173–176. [Google Scholar] [CrossRef]
- Cannio, M.; Ponzoni, C.; Gualtieri, M.L.; Lugli, E.; Leonelli, C.; Romagnoli, M. Stabilization and thermal conductivity of aqueous magnetite nanofluid from continuous flows hydrothermal microwave synthesis. Mater. Lett. 2016, 173, 195–198. [Google Scholar] [CrossRef]
- Özerinç, S.; Kakaç, S.; Yazıcıoğlu, A.G. Enhanced thermal conductivity of nanofluids: A state-of-the-art review. Microfluid. Nanofluid. 2010, 8, 145–170. [Google Scholar] [CrossRef]
- Tang, S.; Liu, Z.Y.; Wang, G.D.; Misra, R.D.K. Microstructural evolution and mechanical properties of high strength microalloyed steels: Ultra Fast Cooling (UFC) versus Accelerated Cooling (ACC). Mater. Sci. Eng. A 2013, 580, 257–265. [Google Scholar] [CrossRef]
- Ginzburg, V.B. Steel-rolling technology. Theory Pract. 1989, 328, 791. [Google Scholar]
- Eghbali, B.; Abdollah-zadeh, A. Influence of deformation temperature on the ferrite grain refinement in a low carbon Nb–Ti microalloyed steel. J. Mater. Process. Technol. 2006, 180, 44–48. [Google Scholar] [CrossRef]
- Raulf, M.; Persson, K. Rolling of Steel. In Encyclopedia of Lubricants and Lubrication; Mang, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1663–1680. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, T.; Erdemir, A.; Li, Q. Tribology of two-dimensional materials: From mechanisms to modulating strategies. Mater. Today 2018, 26, 67–86. [Google Scholar] [CrossRef]
- Song, J.; She, J.; Chen, D.; Pan, F. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloy 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Xie, H.; Jiang, B.; He, J.; Xia, X.; Pan, F. Lubrication performance of MoS2 and SiO2 nanoparticles as lubricant additives in magnesium alloy-steel contacts. Tribol. Int. 2016, 93, 63–70. [Google Scholar] [CrossRef]
- Huang, W.; Du, C.; Li, Z.; Liu, M.; Liu, W. Tribological characteristics of magnesium alloy using N-containing compounds as lubricating additives during sliding. Wear 2006, 260, 140–148. [Google Scholar] [CrossRef]
- Huang, W.; Fu, Y.; Wang, J.; Li, Z.; Liu, M. Effect of chemical structure of borates on the tribological characteristics of magnesium alloy during sliding. Tribol. Int. 2005, 38, 775–780. [Google Scholar] [CrossRef]
- Xia, Y.; Jia, Z.; Jia, J. Tribological Behavior of AZ91D Magnesium Alloy against SAE52100 Steel under Ionic Liquid Lubricated Conditions. In Advanced Tribology; Springer: Berlin, Germany, 2009; pp. 896–898. [Google Scholar]






















| Workpiece & Dimensions | Lubricant | Benchmark | Rolling Temp. | Rolling Reduction | Rolling Speed | Decrease in % | Ref. |
|---|---|---|---|---|---|---|---|
| Mild steel with 30 mm in thickness | 2% anatase TiO2, SHMP | Dry condition & Water | ~950–750 °C | ~81.8% in five passes | - | Up to 20% in the final pass | [273] |
| Mild steel 300 × 50 × 8 mm3 | 0.4–8% TiO2, 0.004–0.08% PEI, 10% glycerol | Dry condition & Water | ~1050–850 °C | ~30% in one pass | 0.35 m/s | Up to 8% | [29] |
| Mild steel 300 × 100 × 8 mm3 | 1–8% TiO2, 0.01–0.08% PEI, 10% glycerol | Dry condition & Water | ~850 °C | ~30% in one pass | 0.35 m/s | Up to 6.8% | [28] |
| Mild steel 300 × 91 × 8.5 mm3 | 2% & 4% TiO2, 0.2% & 0.4% SDBS, 10% glycerol | Water | ~850 °C | ~30% in one pass | 0.35 m/s | Up to 8.3% | [25] |
| Mild steel 100 × 70 × 30 mm3 | MoS2-Al2O3, glycerol, TEOA, SDBS, and SHMP | Base fluid | ~1000–800 °C | ~86.7% in five passes | 1 m/s | Up to 26.9% | [118] |
| Mild steel 300 × 100 × 12 mm3 | 2% & 4% TiO2, 0.1% & 0.2% SDBS, 10% glycerol, 1% Snailcool | Water | ~850 °C | ~27% in one pass | 0.35 m/s | Up to 8.1% | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morshed, A.; Wu, H.; Jiang, Z. A Comprehensive Review of Water-Based Nanolubricants. Lubricants 2021, 9, 89. https://doi.org/10.3390/lubricants9090089
Morshed A, Wu H, Jiang Z. A Comprehensive Review of Water-Based Nanolubricants. Lubricants. 2021; 9(9):89. https://doi.org/10.3390/lubricants9090089
Chicago/Turabian StyleMorshed, Afshana, Hui Wu, and Zhengyi Jiang. 2021. "A Comprehensive Review of Water-Based Nanolubricants" Lubricants 9, no. 9: 89. https://doi.org/10.3390/lubricants9090089