Study on the Quantitative Evaluation of the Surface Force Using a Scanning Probe Microscope
Abstract
:1. Introduction
2. Experimental Approach
2.1. AFM Apparatus
2.2. Substrate
2.3. Oil
2.4. Force Mesurement
3. Results and Discussion
3.1. Effect of Sweep Speed on Force Curve
3.2. Effect of Electrostatic Force on Force Curve
3.3. Effect of Dispersion on Force Curve by Installing and Removing the Chip
3.4. Effect of Elastic Deformation of Substrate on the Force Curve
3.5. Normality of PD Sensitivity Difference Obtained by an Operator
4. Conclusions
- As the sweep speed increases, so does the fluid resistance to a cantilever; in other words, a cantilever detects a repulsive force before contacting the substrate. To keep the non-detecting area of a force curve horizontal, it is necessary to set an appropriate sweep speed according to the spring constant of the cantilever.
- It was demonstrated that the effect of the electrostatic force on the force curve is eliminated by securing a substrate with conductive tape and allowing for charge relaxation for a few hours. In this study, the electrostatic force was eliminated by using carbon tape and letting the sample rest for 60 min.
- The installation position of a cantilever in the holder and the laser position on the cantilever are factors that alter force curves in AFM measurement. In this study, despite the same cantilever, the PD sensitivity was about 1.6 times different with different physical configurations.
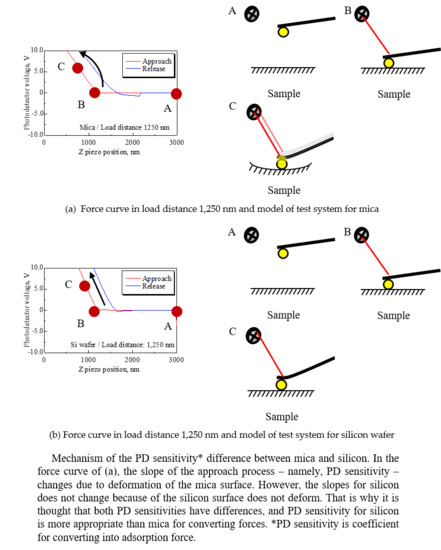
- Two tests using mica and silicon as substrates showed that PD sensitivity is largely influenced by elastic deformation of the substrate. It was suggested that accurate PD sensitivity can be obtained for a cantilever by calibrating the PD sensitivity with a hard substrate like silicon. In this study, a silicon wafer gave an accurate PD sensitivity.
- The distribution of the PD sensitivity deviation (due to repeated reinstallation of the cantilever and laser positioning system) was Gaussian. We proposed a calibration method for obtaining accurate PD sensitivity. In this study, when we set the one-sided permissible error of PD sensitivity as 1.5 nm/V, the theoretical sample size was approximately 100 tests.
Author Contributions
Funding
Conflicts of Interest
References
- Holmberg, K.; Andersson, P.; Erdemir, A. Global energy consumption due to friction in passenger cars. Tribol. Int. 2012, 47, 221–234. [Google Scholar] [CrossRef]
- Qin, L.; Feng, X.; Hafezi, M.; Zhang, Y.; Guo, J.; Dong, G.; Qin, Y. Investigating the tribological and biological performance of covalently grafted chitosan coatings on Co–Cr–Mo alloy. Tribol. Int. 2018, 127, 302–312. [Google Scholar] [CrossRef]
- Soltanahmadi, S.; Esfahani, E.A.; Nedelcu, I.; Morina, A.; van Eijk, M.C.P.; Neville, A. Surface reaction films from amine-based organic friction modifiers and their influence on surface fatigue and friction. Tribol. Lett. 2019, 67, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Guegan, J.; Southby, M.; Spikes, H.A. Friction modifier additives, synergies and antagonisms. Tribol. Lett. 2019, 67, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Campen, S.; Green, J.H.; Lamb, G.D.; Spikes, H.A. In situ study of model organic friction modifiers using liquid cell AFM; saturated and mono-unsaturated carboxylic acids. Tribol. Lett. 2015, 57, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Hirano, F.; Sakai, T.; Kuwano, N.; Ohno, N. Chain matching between hydrocarbon and fatty acid as interfacial phenomena. Tribol. Int. 1987, 20, 186–204. [Google Scholar] [CrossRef]
- Jahanmir, S. Chain length effects in boundary lubrication. Wear 1985, 102, 331–349. [Google Scholar] [CrossRef]
- Hirayama, T.; Kawamura, R.; Fujino, K.; Matsuoka, T.; Komiya, H.; Onishi, H. Cross-sectional imaging of boundary lubrication layer formed by fatty acid by means of frequency-modulation atomic force microscopy. Langmuir 2017, 33, 10492–10500. [Google Scholar] [CrossRef]
- Lundgren, S.M.; Persson, K.; Kronberg, B.; Claesson, P.M. Adsorption of fatty acids from alkane solution studied with quartz crystal microbalance. Tribol. Int. 2006, 22, 15–20. [Google Scholar] [CrossRef]
- Hirayama, T.; Torii, T.; Konishi, Y.; Maeda, M.; Matsuoka, T.; Inoue, K.; Hino, M.; Yamazaki, D.; Takeda, M. Thickness and density of adsorbed additive layer on metal surface in lubricant by neutron reflectometry. Tribol. Int. 2012, 54, 100–105. [Google Scholar] [CrossRef]
- Hirayama, T.; Maeda, M.; Sasaki, Y.; Matsuoka, T.; Komiya, H.; Hino, M. Growth of adsorbed additive layer for further friction reduction. Lubr. Sci. 2019, 31, 171–178. [Google Scholar] [CrossRef]
- Zhu, Y.; Ohtani, H.; Greenfield, M.L.; Ruths, M.; Granick, S. Modification of boundary lubrication by oil-soluble friction modifier additives. Tribol. Lett. 2003, 15, 127–134. [Google Scholar] [CrossRef]
- Bandobashi, T.; Honda, T.; Tamura, K. Analysis of adsorption force of an ashless FM for metal surface by the colloid probe method. Jpn. Soc. Tribol. 2018, 63, 690–698. (In Japanese) [Google Scholar]
- Ducker, W.A.; Senden, T.J.; Pashley, R.M. Measurement of forces in liquids using a force microscope. Langmuir 1992, 8, 1831–1836. [Google Scholar] [CrossRef]
- Van der Hoeven, P.H.C.; Lyklema, J. Electrostatic stabilization in non-aqueous media. Adv. Colloid Interface Sci. 1992, 42, 205–277. [Google Scholar] [CrossRef]
- Kawai, A. Analysis of adsorption force on solid surface with micro tip -influence of surface roughness, surface energy and adsorption water. J. Adhes. Soc. Jpn. 2000, 36, 131–135. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Jones, R.; Pollock, H.M.; Cleaver, J.A.S.; Hodges, C.S. Adhesion force between glass and silicon surfaces in air studied by AFM: Effects of relative humidity, particle size, roughness, and surface treatment. Langmuir 2002, 18, 8045–8055. [Google Scholar] [CrossRef]
- Barbagini, F.; Fyen, W.; Hoeymissen, J.V.; Mertens, P.; Fransaer, J. Time-dependent attractive force between a silica particle and a silica substrate in dodecane. J. Adhes. Sci. Technol. 2008, 22, 65–75. [Google Scholar] [CrossRef]
- Chuang, W.H.; Luger, T.; Fettig, R.K.; Ghodssi, R. Mechanical property characterization of LPCVD silicon nitride thin films at cryogenic temperatures. J. Microelectromech. Syst. 2004, 13, 870–879. [Google Scholar] [CrossRef]
- Morikawa, Y.; Ayukawa, K.; Tsuji, Y. Fluidonics (Japanese), 2nd ed.; Asakura Pubrishing Co., Ltd.: Tokyo, Japan, 1993; p. 81. [Google Scholar]
- Iguchi, Y.; Beak, D.; Momozono, S.; Hemthavy, P.; Saito, S.; Takahashi, K. Adhesional contact between rigid sphere and elastic plane covered with thin liquid film considering contact-angle hysteresis. Tribol. Online 2020, 15, 1–8. [Google Scholar] [CrossRef]
- Matsuoka, H.; Kato, T. Measurement of ultrathin lubrication film thickness in molecular scale. Jpn. Soc. Tribol. 1996, 41, 427–434. (In Japanese) [Google Scholar]
- Wortman, J.J.; Evans, R.A. Young’s modulus, shear modulus, and Poisson’s ratio in silicon and germanium. J. Appl. Phys. 1965, 36, 153–156. [Google Scholar] [CrossRef]
- Williams, D.N.; Williams, K.A.; Monuteaux, M. Statistics in nuclear cardiology: So, what’s the difference? The t-test: Pit falls and options in hypothesis testing for comparing differences in means. Ann. Nucl. Cardiol. 2018, 4, 83–87. [Google Scholar] [CrossRef]

















| Length, µm | 300 |
| Spring constant, N/m | 0.015 |
| Probe material | SiO2 |
| Probe radius, µm | 1 |
| Probe surface | CH3, COOH |
| Specimens | Root Mean Square Rq, nm |
|---|---|
| Mica | 0.34 |
| Silicon wafer | 1.59 |
| Test | PD Sensitivity S, nm/V | |
|---|---|---|
| No. | Long | Short |
| 1 | 54.56 | 92.35 |
| 2 | 54.76 | 92.85 |
| 3 | 54.39 | 91.23 |
| 4 | 54.61 | 91.34 |
| 5 | 55.66 | 93.57 |
| 6 | 54.51 | 91.53 |
| 7 | 54.66 | 92.96 |
| 8 | 55.25 | 90.94 |
| 9 | 55.44 | 92.19 |
| 10 | 55.59 | 92.89 |
| Mean | 54.94 | 92.18 |
| Mean PD Sensitivity S, nm/V | ||
|---|---|---|
| Load distance Ll, nm | Mica | Silicon wafer |
| 1250 | 68.71 | 39.35 |
| 1000 | 61.80 | 36.44 |
| 750 | 72.37 | 36.64 |
| 500 | 72.46 | 163.15 |
| 250 | 130.38 | 264.97 |
| Young’s Modulus, GPa | Poisson’s Ratio | |
|---|---|---|
| Mica 1 | 34.5 | 0.205 |
| Silicon wafer 2 (Silicon monocrystal (110)—plane) | 170 | 0.289 |
| New: Uncalibrated | New: Calibrated | |||
|---|---|---|---|---|
| No. | Adsorption force, nN | No. | Adsorption force, nN | Error bar, nN |
| 1 | 2.861 | 1 | 3.172 | 0.090 |
| 2 | 2.729 | 2 | 3.219 | 0.091 |
| 3 | 2.760 | 3 | 3.202 | 0.091 |
| 4 | 2.767 | 4 | 3.195 | 0.090 |
| 5 | 2.720 | 5 | 3.150 | 0.089 |
| Reference [13]: Uncalibrated | Reference [13]: Calibrated | |||
| No. | Adsorption force, nN | No. | Adsorption force, nN | Error bar, nN |
| 1 | 2.570 | 1 | 3.147 | 0.012 |
| 2 | 2.437 | 2 | 3.137 | 0.012 |
| 3 | 2.369 | 3 | 3.128 | 0.012 |
| 4 | 2.266 | 4 | 3.135 | 0.012 |
| 5 | 2.191 | 5 | 3.091 | 0.012 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yagi, W.; Honda, T.; Tamura, K.; Narita, K. Study on the Quantitative Evaluation of the Surface Force Using a Scanning Probe Microscope. Lubricants 2020, 8, 66. https://doi.org/10.3390/lubricants8060066
Yagi W, Honda T, Tamura K, Narita K. Study on the Quantitative Evaluation of the Surface Force Using a Scanning Probe Microscope. Lubricants. 2020; 8(6):66. https://doi.org/10.3390/lubricants8060066
Chicago/Turabian StyleYagi, Wataru, Tomomi Honda, Kazushi Tamura, and Keiichi Narita. 2020. "Study on the Quantitative Evaluation of the Surface Force Using a Scanning Probe Microscope" Lubricants 8, no. 6: 66. https://doi.org/10.3390/lubricants8060066