Effects of Dry Needling on Active Myofascial Trigger Points and Pain Intensity in Tension-Type Headache: A Randomized Controlled Study
Abstract
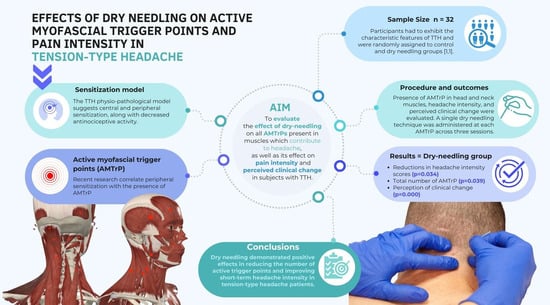
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample
2.3. Measurements
2.3.1. Pain Intensity
2.3.2. MTrP Examination
2.3.3. Perceived Clinical Change
2.4. Intervention
2.5. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashina, S.; Mitsikostas, D.D.; Lee, M.J.; Yamani, N.; Wang, S.-J.; Messina, R.; Ashina, H.; Buse, D.C.; Pozo-Rosich, P.; Jensen, R.H.; et al. Tension-type headache. Nat. Rev. Dis. Prim. 2021, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Onan, D.; Younis, S.; Wellsgatnik, W.D.; Farham, F.; Andruškevičius, S.; Abashidze, A.; Jusupova, A.; Romanenko, Y.; Grosu, O.; Moldokulova, M.Z.; et al. Debate: Differences and similarities between tension-type headache and migraine. J. Headache Pain 2023, 24, 92. [Google Scholar] [CrossRef] [PubMed]
- The World Health Organization & Lifting the Burden. Atlas of Headache Disorders and Resources in the World 2011; World Health Organization: Geneva, Switzerland, 2011; Volume 72.
- Linde, M.; Gustavsson, A.; Stovner, L.J.; Steiner, T.J.; Barré, J.; Katsarava, Z.; Lainez, J.M.; Lampl, C.; Lantéri-Minet, M.; Rastenyte, D.; et al. The cost of headache disorders in Europe: The Eurolight project. Eur. J. Neurol. 2012, 19, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Sabah, Z.U.; Aziz, S.; Narapureddy, B.R.; Alasiri, H.A.A.; Asiri, H.Y.M.; Asiri, A.H.H.; Alsulami, A.A.H.; Hassan, N.K.A.; Mohammed Asif, S.; Alsyd, S.M. Clinical-Epidemiology of Tension-Type Headache among the Medical and Dental Undergraduates of King Khalid University, Abha, Saudi Arabia. J. Pers. Med. 2022, 12, 2064. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Bendtsen, L.; Schoenen, J. Synthesis of tension type headache mechanisms. In The Headaches; Olesen, J., Goadsby, P.J., Ramadan, N.M., Tfelt-Hansen, P., Welch, K.M.A., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; Volume 3, pp. 679–683. [Google Scholar]
- Romero-Godoy, R.; Romero-Godoy, S.R.; Romero-Acebal, M.; Gutiérrez-Bedmar, M. Sensory Thresholds and Peripheral Nerve Responses in Chronic Tension-Type Headache and Neuropsychological Correlation. J. Clin. Med. 2022, 11, 1905. [Google Scholar] [CrossRef]
- Ricci, V.; Ricci, C.; Mezian, K.; Naňka, O.; Özçakar, L. Trapezius Muscle and the Cutaneous Branches of Spinal Nerves: Sonographic/Anatomic Discussion of Myofascial Pain and Superficial Injections. Pain Med. 2023, 24, 221–225. [Google Scholar] [CrossRef]
- Palacios-Ceña, M.; Wang, K.; Castaldo, M.; Guillem-Mesado, A.; Ordás-Bandera, C.; Arendt-Nielsen, L.; Fernández-de-las-Peñas, C. Trigger points are associated with widespread pressure pain sensitivity in people with tension-type headache. Cephalalgia 2018, 38, 237–245. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Cuadrado, M.L.; Arendt-Nielsen, L.; Simons, D.G.; Pareja, J.A. Myofascial trigger points and sensitization: An updated pain model for tension-type headache. Cephalalgia 2007, 27, 383–393. [Google Scholar] [CrossRef]
- Ezpeleta, D.; Pozo-Rosich, P. Guía Oficial de Práctica Clínica en Cefaleas; Luzán 5: Madrid, Spain, 2015; ISBN 9788415198994. [Google Scholar]
- Edvinsson, J.C.A.; Viganò, A.; Alekseeva, A.; Alieva, E.; Arruda, R.; De Luca, C.; D’Ettore, N.; Frattale, I.; Kurnukhina, M.; Macerola, N.; et al. The fifth cranial nerve in headaches. J. Headache Pain 2020, 21, 65. [Google Scholar] [CrossRef]
- Nöbel, M.; Feistel, S.; Ellrich, J.; Messlinger, K. ATP-sensitive muscle afferents activate spinal trigeminal neurons with meningeal afferent input in rat—Pathophysiological implications for tension-type headache. J. Headache Pain 2016, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Fernández-De-Las-Peñas, C.; Alonso-Blanco, C.; Cuadrado, M.L.; Gerwin, R.D.; Pareja, J.A.; Fernández-De-Las-Peñas, C.; Alonso-Blanco, C.; Cuadrado, M.L.; Gerwin, R.D.; Pareja, J.A. Trigger points in the suboccipital muscles and forward head posture in tension-type headache. Headache 2006, 46, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Do, T.P.; Heldarskard, G.F.; Kolding, L.T.; Hvedstrup, J.; Schytz, H.W. Myofascial trigger points in migraine and tension-type headache. J. Headache Pain 2018, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Fernandez De Las Peñas, C.; Cuadrado, M.L.; Gerwin, R.D.; Pareja, J.A. Referred pain from the trochlear region in tension-type headache: A myofascial trigger point from the superior oblique muscle. Headache 2005, 45, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Ge, H.Y.; Arendt-Nielsen, L.; Cuadrado, M.L.; Pareja, J.A. The local and referred pain from myofascial trigger points in the temporalis muscle contributes to pain profile in chronic tension-type headache. Clin. J. Pain 2007, 23, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Ge, H.Y.; Alonso-Blanco, C.; González-Iglesias, J.; Arendt-Nielsen, L. Referred pain areas of active myofascial trigger points in head, neck, and shoulder muscles, in chronic tension type headache. J. Bodyw. Mov. Ther. 2010, 14, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.M.; Fernández de las Peñas, C.; Simons, D.G.; Cummings, B.D.; Jiménez González, D.; Travell, J.G.; Simons, L.S. Travell‚ Simons & Simons Dolor y Disfunción Miofascial. In El Manual de los Puntos Gatillo; Wolters Kluwer: Philadelphia, PA, USA, 2019; p. 935. [Google Scholar]
- Alnasser, A.; Alhumrran, H.; Alfehaid, M.; Alhamoud, M.; Albunaian, N.; Ferwana, M. Paracetamol versus ibuprofen in treating episodic tension-type headache: A systematic review and network meta-analysis. Sci. Rep. 2023, 13, 21532. [Google Scholar] [CrossRef] [PubMed]
- Banzi, R.; Cusi, C.; Randazzo, C.; Sterzi, R.; Tedesco, D.; Moja, L. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of tension-type headache in adults. Cochrane Database Syst. Rev. 2015, 2015, CD011681. [Google Scholar] [CrossRef]
- Monzani, L.; Espí-López, G.V.; Zurriaga, R.; Andersen, L.L. Manual therapy for tension-type headache related to quality of work life and work presenteeism: Secondary analysis of a randomized controlled trial. Complement. Ther. Med. 2016, 25, 86–91. [Google Scholar] [CrossRef]
- Jung, A.; Eschke, R.C.; Struss, J.; Taucher, W.; Luedtke, K. Effectiveness of physiotherapy interventions on headache intensity, frequency, duration and quality of life of patients with tension-type headache. A systematic review and network meta-analysis. Cephalalgia 2022, 42, 944–965. [Google Scholar] [CrossRef]
- Grazzi, L.; Grignani, E.; Sansone, E.; Láinez, M.J.A.; García-Ull, J. Neuromodulation and Other Non-pharmacological Approaches in Tension-Type Headache BT—Neuromodulation in Headache and Facial Pain Management: Principles, Rationale and Clinical Data; Lambru, G., Lanteri-Minet, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 157–172. ISBN 978-3-030-14121-9. [Google Scholar]
- Holroyd, K.A.; Martin, P.R.; Nash, J.M. Psychological Treatments of Tension-Type Headaches. In The Headaches; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; pp. 711–719. [Google Scholar]
- Lee, H.J.; Lee, J.H.; Cho, E.Y.; Kim, S.M.; Yoon, S. Efficacy of psychological treatment for headache disorder: A systematic review and meta-analysis. J. Headache Pain 2019, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Grazzi, L.; Toppo, C.; D’Amico, D.; Leonardi, M.; Martelletti, P.; Raggi, A.; Guastafierro, E. Non-Pharmacological Approaches to Headaches: Non-Invasive Neuromodulation, Nutraceuticals, and Behavioral Approaches. Int. J. Environ. Res. Public Health 2021, 18, 1503. [Google Scholar] [CrossRef]
- Shahverdi, Z.A.; Dehghani, M.; Ashouri, A.; Manouchehri, M.; Mohebi, N. Effectiveness of intensive short-term dynamic psychotherapy for Tension-Type Headache (TTH): A randomized controlled trial of effects on emotion regulation, anger, anxiety, and TTH symptom severity. Acta Psychol. 2024, 244, 104176. [Google Scholar] [CrossRef]
- Vázquez-Justes, D.; Yarzábal-Rodríguez, R.; Doménech-García, V.; Herrero, P.; Bellosta-López, P. Effectiveness of dry needling for headache: A systematic review. Neurologia 2022, 37, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Pi, C.; Liu, Y.; Li, L.; Tang, W.; Yan, X.; Yu, S. Effects on neuromodulation, acupuncture, and aerobic exercises on migraine and tension-type headache outcomes: A systematic review and meta-analysis. Medicine 2022, 101, e30530. [Google Scholar] [CrossRef]
- Gerber, L.H.; Shah, J.; Rosenberger, W.; Armstrong, K.; Turo, D.; Otto, P.; Heimur, J.; Thaker, N.; Sikdar, S. Dry Needling Alters Trigger Points in the Upper Trapezius Muscle and Reduces Pain in Subjects With Chronic Myofascial Pain. PM&R 2015, 7, 711–718. [Google Scholar] [CrossRef]
- Pourahmadi, M.; Mohseni-Bandpei, M.A.; Keshtkar, A.; Koes, B.W.; Fernández-De-Las-Peñas, C.; Dommerholt, J.; Bahramian, M. Effectiveness of dry needling for improving pain and disability in adults with tension-type, cervicogenic, or migraine headaches: Protocol for a systematic review. Chiropr. Man. Ther. 2019, 27, 43. [Google Scholar] [CrossRef] [PubMed]
- Gildir, S.; Tüzün, E.H.; Eroglu, G.; Eker, L. A randomized trial of trigger point dry needling versus sham needling for chronic tension-type headache. Medicine 2019, 98, e14520. [Google Scholar] [CrossRef]
- Kamali, F.; Mohamadi, M.; Fakheri, L.; Mohammadnejad, F. Dry needling versus friction massage to treat tension type headache: A randomized clinical trial. J. Bodyw. Mov. Ther. 2019, 23, 89–93. [Google Scholar] [CrossRef]
- Berggreen, S.; Wiik, E.; Lund, H. Treatment of myofascial trigger points in female patients with chronic tension-type headache—A randomized controlled trial. Adv. Physiother. 2012, 14, 10–17. [Google Scholar] [CrossRef]
- Simons, D.G.; Travell, J.G. Dolor y Disfuncion Miofascial: El Manual de los Puntos Gatillo, 3rd ed.; Donnelly, J.M., Fernández de las Peñas, C., Simons, D.G., Simons, L.S., Travell, J.G., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2019; ISBN 9788417602024. [Google Scholar]
- Ferreira-Valente, M.A.; Pais-Ribeiro, J.L.; Jensen, M.P. Validity of four pain intensity rating scales. Pain 2011, 152, 2399–2404. [Google Scholar] [CrossRef] [PubMed]
- Stratford, P.W.; Binkley, J.M.; Riddle, D.L. Health status measures: Strategies and analytic methods for assessing change scores. Phys. Ther. 1996, 76, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Bobos, P.; MacDermid, J.; Nazari, G.; Furtado, R. Psychometric properties of the global rating of change scales in patients with neck disorders: A systematic review with meta-analysis and meta-regression. BMJ Open 2019, 9, e033909. [Google Scholar] [CrossRef]
- Roch, M.; Morin, M.; Gaudreault, N. Immediate Effect of Dry Needling on the Viscoelastic Properties of a Trigger Point on the Infraspinatus Muscle Measured with MyotonPRO. Physiother. Can. 2022, 74, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Adigozali, H.; Shadmehr, A.; Ebrahimi, E.; Rezasoltani, A.; Naderi, F. B mode, doppler and ultrasound elastography imaging on active trigger point in women with myofascial pain syndrome treated by dry needling. Muscles Ligaments Tendons J. 2019, 9, 417–424. [Google Scholar] [CrossRef]
- Baldry, P.E.; Thompson, J.W. Chapter 10—Treatment of myofascial trigger point pain and fibromyalgia syndromes. In Trigger Points and Musculoskeletal Pain, 3rd ed.; Baldry, P.E., Thompson, J.W.B.T.-A., Eds.; Churchill Livingstone: Edinburgh, UK, 2005; pp. 127–148. ISBN 978-0-443-06644-3. [Google Scholar]
- Chen, J.T.; Chung, K.C.; Hou, C.R.; Kuan, T.S.; Chen, S.M.; Hong, C.Z. Inhibitory effect of dry needling on the spontaneous electrical activity recorded from myofascial trigger spots of rabbit skeletal muscle. Am. J. Phys. Med. Rehabil. 2001, 80, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-L.; Chou, L.-W.; Joe, Y.-S.; Hong, C.-Z. Spinal cord mechanism involving the remote effects of dry needling on the irritability of myofascial trigger spots in rabbit skeletal muscle. Arch. Phys. Med. Rehabil. 2011, 92, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Sato, Y.; Shimura, M.; Uchida, S. Calcitonin gene-related peptide produces skeletal muscle vasodilation following antidromic stimulation of unmyelinated afferents in the dorsal root in rats. Neurosci. Lett. 2000, 283, 137–140. [Google Scholar] [CrossRef]
- Shah, J.P.; Gilliams, E.A. Uncovering the biochemical milieu of myofascial trigger points using in vivo microdialysis: An application of muscle pain concepts to myofascial pain syndrome. J. Bodyw. Mov. Ther. 2008, 12, 371–384. [Google Scholar] [CrossRef]
- Hsieh, Y.-L.; Yang, S.-A.; Yang, C.-C.; Chou, L.-W. Dry Needling at Myofascial Trigger Spots of Rabbit Skeletal Muscles Modulates the Biochemicals Associated with Pain, Inflammation, and Hypoxia. Evid.-Based Complement. Altern. Med. 2012, 2012, 342165. [Google Scholar] [CrossRef]
- Margalef, R.; Sisquella, M.; Bosque, M.; Romeu, C.; Mayoral, O.; Monterde, S.; Priego, M.; Guerra-Perez, R.; Ortiz, N.; Tomàs, J.; et al. Experimental myofascial trigger point creation in rodents. J. Appl. Physiol. 2018, 126, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Chou, L.-W.; Kao, M.-J.; Lin, J.-G. Probable mechanisms of needling therapies for myofascial pain control. Evid.-Based Complement. Altern. Med. 2012, 2012, 705327. [Google Scholar] [CrossRef] [PubMed]
- Cagnie, B.; Dewitte, V.; Barbe, T.; Timmermans, F.; Delrue, N.; Meeus, M. Physiologic Effects of Dry Needling. Curr. Pain Headache Rep. 2013, 17, 348. [Google Scholar] [CrossRef] [PubMed]
- Langevin, H.M. Acupuncture, connective tissue, and peripheral sensory modulation. Crit. Rev. Eukaryot. Gene Expr. 2014, 24, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Langevin, H.M.; Churchill, D.L.; Cipolla, M.J. Mechanical signaling through connective tissue: A mechanism for the therapeutic effect of acupuncture. FASEB J. 2001, 15, 2275–2282. [Google Scholar] [CrossRef] [PubMed]
- Karakurum, B.; Karaalin, O.; Coskun, Ö.; Dora, B.; Üçler, S.; Inan, L.E. The “dry-needle technique”: Intramuscular stimulation in tension-type headache. Cephalalgia 2001, 21, 813–817. [Google Scholar] [CrossRef] [PubMed]
- García-de la-Banda-García, R.; Cortés-Pérez, I.; Ibancos-Losada, M.D.R.; López-Ruiz, M.D.C.; Obrero-Gaitán, E.; Osuna-Pérez, M.C. Effectiveness of Dry Needling versus Manual Therapy in Myofascial Temporomandibular Disorders: A Single-Blind Randomized Controlled Trial. J. Pers. Med. 2023, 13, 1415. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sacristán, L.; Calvo-Lobo, C.; Pecos-Martín, D.; Fernández-Carnero, J.; Alonso-Pérez, J.L. Dry needling in active or latent trigger point in patients with neck pain: A randomized clinical trial. Sci. Rep. 2022, 12, 3188. [Google Scholar] [CrossRef]
- Mousavi-Khatir, S.R.; Fernández-de-Las-Peñas, C.; Saadat, P.; Javanshir, K.; Zohrevand, A. The Effect of Adding Dry Needling to Physical Therapy in the Treatment of Cervicogenic Headache: A Randomized Controlled Trial. Pain Med. 2022, 23, 579–589. [Google Scholar] [CrossRef]
- Pandya, J.; Puentedura, E.J.; Koppenhaver, S.; Cleland, J. Dry needling versus Manual Therapy in patients with Mechanical Neck Pain: A Randomized Control Trial. J. Orthop. Sports Phys. Ther. 2024, 54, 1–46. [Google Scholar] [CrossRef]
- Dunning, J.; Butts, R.; Zacharko, N.; Fandry, K.; Young, I.; Wheeler, K.; Day, J.; Fernández-de-las-Peñas, C. Spinal manipulation and perineural electrical dry needling in patients with cervicogenic headache: A multicenter randomized clinical trial. Spine J. 2021, 21, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas-Barea, S.; Pérez-Guillén, S.; López-De-Celis, C.; Rodríguez-Sanz, J.; Fanlo-Mazas, P.; Carrasco-Uribarren, A. Effects of diacutaneous fibrolysis in patients with tension-type headache: A randomized controlled trial. PLoS ONE 2023, 18, e0273877. [Google Scholar] [CrossRef] [PubMed]
- Moraska, A.F.; Stenerson, L.; Butryn, N.; Krutsch, J.P.; Schmiege, S.J.; Mann, J.D. Myofascial trigger point-focused head and neck massage for recurrent tension-type headache: A randomized, placebo-controlled clinical trial. Clin. J. Pain 2015, 31, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Kamper, S. Global rating of change scales. Aust. J. Physiother. 2009, 55, 289. [Google Scholar] [CrossRef] [PubMed]
- Martín-Pintado-Zugasti, A.; Mayoral Del Moral, O.; Gerwin, R.D.; Fernández-Carnero, J. Post-needling soreness after myofascial trigger point dry needling: Current status and future research. J. Bodyw. Mov. Ther. 2018, 22, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Krøll, L.S.; Callesen, H.E.; Carlsen, L.N.; Birkefoss, K.; Beier, D.; Christensen, H.W.; Jensen, M.; Tómasdóttir, H.; Würtzen, H.; Høst, C.V.; et al. Manual joint mobilisation techniques, supervised physical activity, psychological treatment, acupuncture and patient education for patients with tension-type headache. A systematic review and meta-analysis. J. Headache Pain 2021, 22, 96. [Google Scholar] [CrossRef] [PubMed]
- Pourahmadi, M.; Dommerholt, J.; Fernández-de-Las-Peñas, C.; Koes, B.W.; Mohseni-Bandpei, M.A.; Mansournia, M.A.; Delavari, S.; Keshtkar, A.; Bahramian, M. Dry Needling for the Treatment of Tension-Type, Cervicogenic, or Migraine Headaches: A Systematic Review and Meta-Analysis. Phys. Ther. 2021, 101, pzab068. [Google Scholar] [CrossRef]


| All the Sample | Control Group | Intervention Group | p Value | |
|---|---|---|---|---|
| Age (years) | 39.09 ± 12.68 | 41.44 ± 14.68 | 31.75 ± 10.75 | 0.308 ** |
| Headache frequency (days/month) | 13.44 ± 10.19 | 13.19 ± 11.55 | 13.69 ± 9.00 | 0.569 ** |
| Headache medication (doses/month) | 29.38 ± 53.73 | 39.81 ± 69.98 | 18.94 ± 28.21 | 0.227 ** |
| VAS (mm) | 19.31 ± 18.75 | 19.44 ± 19.07 | 19.19 ± 19.05 | 0.955 ** |
| Total number of AMTrPs | 15.53 ± 8.21 | 13.50 ± 8.02 | 17.56 ± 8.15 | 0.165 * |
| Control Group | Intervention Group | p Value | |
|---|---|---|---|
| Headache medication (doses/month) | 46.63 ± 72.54 | 13.00 ± 25.03 | 0.009 ** |
| VAS (mm) | 27.93 ± 31.52 | 10.50 ± 16.79 | 0.160 ** |
| Total number of AMTrPs | 15.86 ± 7.83 | 13.25 ± 9.47 | 0.402 * |
| GROC | −0.38 ± 2.28 | 3.94 ± 2.08 | 0.000 ** |
| Dependent Variable: VAS (mm) | Mean Difference (Control Less Intervention Group) | Confidence Interval for the Difference | p Value |
|---|---|---|---|
| Post-treatment | 23.45 | 1.89–45.01 | 0.034 |
| Dependent Variable: Total Number of AMTrPs | Mean Difference (Pre Less Post-Treatment) | Confidence Interval for the Difference | p Value |
|---|---|---|---|
| Intervention | 4.50 | 0.25–8.75 | 0.039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monti-Ballano, S.; Márquez-Gonzalvo, S.; Lucha-López, M.O.; Ferrández-Laliena, L.; Vicente-Pina, L.; Sánchez-Rodríguez, R.; Tricás-Vidal, H.J.; Tricás-Moreno, J.M. Effects of Dry Needling on Active Myofascial Trigger Points and Pain Intensity in Tension-Type Headache: A Randomized Controlled Study. J. Pers. Med. 2024, 14, 332. https://doi.org/10.3390/jpm14040332
Monti-Ballano S, Márquez-Gonzalvo S, Lucha-López MO, Ferrández-Laliena L, Vicente-Pina L, Sánchez-Rodríguez R, Tricás-Vidal HJ, Tricás-Moreno JM. Effects of Dry Needling on Active Myofascial Trigger Points and Pain Intensity in Tension-Type Headache: A Randomized Controlled Study. Journal of Personalized Medicine. 2024; 14(4):332. https://doi.org/10.3390/jpm14040332
Chicago/Turabian StyleMonti-Ballano, Sofía, Sergio Márquez-Gonzalvo, María Orosia Lucha-López, Loreto Ferrández-Laliena, Lucía Vicente-Pina, Rocío Sánchez-Rodríguez, Héctor José Tricás-Vidal, and José Miguel Tricás-Moreno. 2024. "Effects of Dry Needling on Active Myofascial Trigger Points and Pain Intensity in Tension-Type Headache: A Randomized Controlled Study" Journal of Personalized Medicine 14, no. 4: 332. https://doi.org/10.3390/jpm14040332