Comparison of the Incidence of Postoperative Acute Kidney Injury Following the Administration of Remimazolam or Sevoflurane in Elderly Patients Undergoing Total Knee Arthroplasty: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Methods and Materials
2.1. Ethics and Study Design
2.2. Participants
2.3. Outcomes
2.4. Blind and Randomization
2.5. Procedures
2.6. Data Collection and Outcome Assessment
2.7. Sample Size
2.8. Statistical Analysis
3. Results
3.1. Study Population and Baseline Characteristics
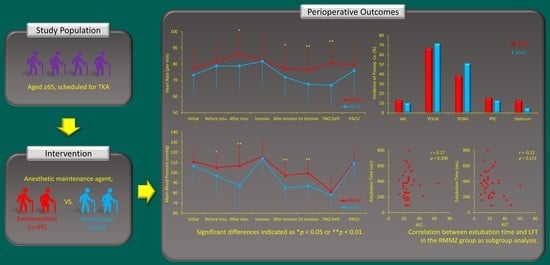
3.2. Primary Outcome
3.3. Secondary Outcomes
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakai, W.; Yoshikawa, Y.; Hirata, N.; Yamakage, M. Effect of remifentanil during cardiopulmonary bypass on incidence of acute kidney injury after cardiac surgery. J. Anesth. 2017, 31, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Biteker, M.; Dayan, A.; Tekkeşin, A.İ.; Can, M.M.; Taycı, İ.; Ilhan, E.; Şahin, G. Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am. J. Surg. 2014, 207, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Mizota, T.; Yamamoto, Y.; Hamada, M.; Matsukawa, S.; Shimizu, S.; Kai, S. Intraoperative oliguria predicts acute kidney injury after major abdominal surgery. Br. J. Anaesth. 2017, 119, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Oh, A.-Y.; Koo, C.-H.; Bae, Y.K.; Jeon, Y.-T. Effects of Anesthetic Technique on the Occurrence of Acute Kidney Injury after Spine Surgery: A Retrospective Cohort Study. J. Clin. Med. 2021, 10, 5653. [Google Scholar] [CrossRef]
- Finlay, S.; Bray, B.; Lewington, A.; Hunter-Rowe, C.; Banerjee, A.; Atkinson, J.; Jones, M. Identification of risk factors associated with acute kidney injury in patients admitted to acute medical units. Clin. Med. 2013, 13, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.H.; Jun, I.-G.; Moon, Y.-J.; Jeon, A.R.; Kim, S.-H.; Kim, B.; Song, J.-G. Association of Preoperative Prognostic Nutritional Index and Postoperative Acute Kidney Injury in Patients Who Underwent Hepatectomy for Hepatocellular Carcinoma. J. Pers. Med. 2021, 11, 428. [Google Scholar] [CrossRef]
- Kane, R.L.; Saleh, K.J.; Wilt, T.J.; Bershadsky, B. The functional outcomes of total knee arthroplasty. J. Bone Jt. Surg. Am. 2005, 87, 1719–1724. [Google Scholar]
- Kim, H.J.; Park, H.S.; Go, Y.J.; Koh, W.U.; Kim, H.; Song, J.G.; Ro, Y.J. Effect of Anesthetic Technique on the Occurrence of Acute Kidney Injury after Total Knee Arthroplasty. J. Clin. Med. 2019, 8, 778. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Koh, W.U.; Kim, S.G.; Park, H.S.; Song, J.G.; Ro, Y.J.; Yang, H.S. Early postoperative albumin level following total knee arthroplasty is associated with acute kidney injury: A retro-spective analysis of 1309 consecutive patients based on kidney disease improving global outcomes criteria. Medicine 2016, 95, e4489–e4495. [Google Scholar] [CrossRef]
- Kim, K.M. Remimazolam: Pharmacological characteristics and clinical applications in anesthesiology. Anesth. Pain Med. 2022, 17, 1–11. [Google Scholar] [CrossRef]
- Kilpatrick, G.J. Remimazolam: Non-Clinical and Clinical Profile of a New Sedative/Anesthetic Agent. Front. Pharmacol. 2021, 12, 690875. [Google Scholar] [CrossRef]
- Kim, S.-H.; Fechner, J. Remimazolam—Current knowledge on a new intravenous benzodiazepine anesthetic agent. Korean J. Anesthesiol. 2022, 75, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Sigel, E.; Buhr, A. The benzodiazepine binding site of GABAA receptors. Trends Pharmacol. Sci. 1997, 18, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Worthington, M.T.; Antonik, L.J.; Goldwater, D.R.; Lees, J.P.; Wilhelm-Ogunbiyi, K.; Borkett, K.M.; Mitchell, M.C. A Phase Ib, Dose-Finding Study of Multiple Doses of Remimazolam (CNS 7056) in Volunteers Undergoing Colonoscopy. Obstet. Anesth. Dig. 2013, 117, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Kim, M.H.; Kong, H.J.; Shin, H.J.; Yang, S.; Kim, N.Y.; Chae, D. Effects of Remimazolam vs. Sevoflurane Anesthesia on Intraoperative Hemodynamics in Patients with Gastric Cancer Undergoing Robotic Gastrectomy: A Propensity Score-Matched Analysis. J. Clin. Med. 2022, 11, 2643. [Google Scholar] [CrossRef]
- Qiu, Y.; Gu, W.; Zhao, M.; Zhang, Y.; Wu, J. The hemodynamic stability of remimazolam compared with propofol in patients undergoing endoscopic submucosal dissection: A randomized trial. Front. Med. 2022, 9, 938940. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Lai, T.; Chen, J.; Lu, Y.; He, F.; Chen, Y.; Xie, Y. Effect of remimazolam induction on hemodynamics in patients undergoing valve replacement surgery: A randomized, double-blind, controlled trial. Pharmacol. Res. Perspect. 2021, 9, e00851. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Heo, H.-J.; Lee, J.-H.; Cho, H.-G.; Kim, G. Assessing the Safety of Total Intravenous Anesthesia with Remimazolam in General Anesthesia for Transcatheter Aortic Valve Implantation of Severe Aortic Valve Stenosis: A Case Series. Medicina 2022, 58, 1680. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pr. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Aldrete, J. The post-anesthesia recovery score revisited. J. Clin. Anesth. 1995, 7, 89–91. [Google Scholar] [CrossRef]
- Serlin, D.C.; Heidelbaugh, J.J.; Stoffel, J. Urinary Retention in Adults: Evaluation and Initial Management. Am. Fam. Physician 2018, 98, 496–503. [Google Scholar] [PubMed]
- Stoffel, J.T.; Peterson, A.C.; Sandhu, J.; Suskind, A.M.; Wei, J.T.; Lightner, D.J. AUA White Paper on Nonneurogenic Chronic Urinary Retention: Consensus Definition, Treatment Algorithm, and Outcome End Points. J. Urol. 2017, 198, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Miskovic, A.; Lumb, A.B. Postoperative pulmonary complications. Br. J. Anaesth. 2017, 118, 317–334. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, H.S.; Kim, J.Y.; Han, D.W.; Yang, J.Y.; Kim, M.J.; Song, Y. Comparison of remimazolam-based and propofol-based total intravenous anesthesia on postoperative quality of re-covery: A randomized non-inferiority trial. J. Clin. Anesth. 2022, 82, 110955. [Google Scholar] [CrossRef]
- Song, S.W.; Jang, Y.N.; Yoon, M.-W.; Jeon, Y.-G. Quality of recovery in patients administered remimazolam versus those administered an inhalant agent for the maintenance of general anesthesia: A randomized control trial. BMC Anesthesiol. 2022, 22, 226. [Google Scholar] [CrossRef]
- Chang, Y.; Chi, K.-Y.; Tai, T.-W.; Cheng, Y.-S.; Lee, P.-H.; Huang, C.-C.; Lee, J.-S. Risk factors for postoperative urinary retention following elective spine surgery: A meta-analysis. Spine J. 2021, 21, 1802–1811. [Google Scholar] [CrossRef]
- Cha, J.E.; Park, S.W.; Choi, Y.I.; Oh, I.D.; Kang, H.Y.; Lee, S.H.; Choi, J.H. Sugammadex use can decrease the incidence of post-operative urinary retention by avoiding anticholinergics: A retro-spective study. Anesth. Pain Med. 2018, 13, 40–46. [Google Scholar] [CrossRef]
- Elbakry, A.E.; Sultan, W.E.; Ibrahim, E. A comparison between inhalational (Desflurane) and total intravenous anaesthesia (Propofol and dexmedetomidine) in improving postoperative recovery for morbidly obese patients undergoing laparoscopic sleeve gas-trectomy: A double-blinded randomised controlled trial. J. Clin. Anesth. 2018, 45, 6–11. [Google Scholar] [PubMed]
- Aoki, Y.; Kurita, T.; Nakajima, M.; Imai, R.; Suzuki, Y.; Makino, H.; Kinoshita, H.; Doi, M.; Nakajima, Y. Association between remimazolam and postoperative delirium in older adults undergoing elective cardiovascular surgery: A prospective cohort study. J. Anesth. 2023, 37, 13–22. [Google Scholar] [CrossRef]
- Kaneko, S.; Morimoto, T.; Ichinomiya, T.; Murata, H.; Yoshitomi, O.; Hara, T. Effect of remimazolam on the incidence of delirium after transcatheter aortic valve implantation under general anes-thesia: A retrospective exploratory study. J. Anesth. 2023, 37, 210–218. [Google Scholar] [CrossRef]
- Yang, X.; Lin, C.; Chen, S.; Huang, Y.; Cheng, Q.; Yao, Y. Remimazolam for the Prevention of Emergence Delirium in Children Following Tonsillectomy and Adenoidectomy Under Sevoflurane Anesthesia: A Randomized Controlled Study. Drug Des. Dev. Ther. 2022, 16, 3413–3420. [Google Scholar] [CrossRef]
- Stöhr, T.; Colin, P.J.; Ossig, J.; Pesic, M.; Borkett, K.; Winkle, P.; Struys, M.M.; Schippers, F. Pharmacokinetic properties of remimazolam in subjects with hepatic or renal impairment. Br. J. Anaesth. 2021, 127, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, H.; Adachi, Y.; Arimura, S.; Kanno, M.; Satoh, T. Compound A concentrations during low-flow sevoflurane anesthesia correlate directly with the concentration of monovalent bases in carbon dioxide absorbents. Anesth. Analg. 2000, 91, 434–439. [Google Scholar] [PubMed]
- Kharasch, E.D.; Schroeder, J.L.; Sheffels, P.; Liggitt, H.D. Influence of sevoflurane on the metabolism and renal effects of compound A in rats. Anesthesiology 2005, 103, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Gonsowski, C.T.; Laster, M.J.; Eger, E.I., 2nd; Ferrell, L.D.; Kerschmann, R.L. Toxicity of compound A in rats. Effect of a 3-hour administration. Anesthesiology 1994, 80, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Obata, R.; Bito, H.; Ohmura, M.; Moriwaki, G.; Ikeuchi, Y.; Katoh, T.; Sato, S. The effects of prolonged low-flow sevoflurane anesthesia on renal and hepatic function. Anesth. Analg. 2000, 91, 1262–1268. [Google Scholar]
- Kharasch, E.D.; Frink, E.J.; Artru, A.; Michalowski, P.; Rooke, G.A.; Nogami, W. Long-Duration Low-Flow Sevoflurane and Isoflurane Effects on Postoperative Renal and Hepatic Function. Obstet. Anesth. Dig. 2001, 93, 1511–1520. [Google Scholar] [CrossRef]
- Park, M.; Jung, K.; Cho, H.S.; Min, J.-J. Renal injury from sevoflurane in noncardiac surgery: A retrospective cohort study. Br. J. Anaesth. 2022, 129, 182–190. [Google Scholar] [CrossRef]
- Zhou, J.; Leonowens, C.; Ivaturi, V.D.; Lohmer, L.L.; Curd, L.; Ossig, J.; Schippers, F.; Petersen, K.-U.; Stoehr, T.; Schmith, V. Population pharmacokinetic/pharmacodynamic modeling for remimazolam in the induction and maintenance of general anesthesia in healthy subjects and in surgical subjects. J. Clin. Anesth. 2020, 66, 109899. [Google Scholar] [CrossRef]
- Park, I.; Cho, M.; Nam, S.W.; Hwang, J.-W.; Do, S.-H.; Na, H.-S. Total intravenous anesthesia induced and maintained by a combination of remimazolam and remifentanil without a neuromuscular blocking agent: A prospective, observational pilot study. BMC Anesth. 2022, 22, 237. [Google Scholar] [CrossRef]
- Matsumoto, H.; Okuno, M.; Nakamura, T.; Yamamoto, K.; Hagino, H. Fall incidence and risk factors in patients after total knee arthroplasty. Arch. Orthop. Trauma Surg. 2012, 132, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.O.; Pearson, M.; Latham, S.K. Are people following hip and knee arthroplasty at greater risk of experiencing a fall and fracture? Data from the Os-teoarthritis Initiative. Arch. Orthop. Trauma Surg. 2016, 136, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Koh, I.J.; Kim, T.K.; Chang, C.B.; Cho, H.J.; In, Y. Trends in use of total knee arthroplasty in Korea from 2001 to 2010. Clin. Orthop. Relat. Res. 2013, 471, 1441–1450. [Google Scholar] [CrossRef] [PubMed]



| RMMZ (n = 39) | SEVO (n = 39) | p-Value | |
|---|---|---|---|
| Age (yr) | 75 (71–78) | 72 (69–78) | 0.298 |
| Female (n) | 33 (84.6%) | 34 (87.2%) | 1.000 |
| Body mass index (kg/m2) | 24.3 (22.5–26.6) | 23.6 (22.4–27.3) | 0.826 |
| ASA-PS class (I/II/III) | 3 (7.7%) /33 (84.6%) / 3 (7.7%) | 7 (17.9%)/30 (76.9%)/2 (5.1%) | 0.379 |
| Diabetes | 13 (33.3%) | 9 (23.1%) | 0.450 |
| Hypertension | 32 (82.1%) | 30 (76.9%) | 0.779 |
| Chronic kidney disease | 4 (10.3%) | 2 (5.1%) | 0.671 |
| Hematocrit (%) | 39.7 (37.2–41.5) | 39.5 (37.5–41.7) | 0.877 |
| Platelet (×103/μL) | 238 (199–263) | 222 (205–249) | 0.675 |
| INR | 0.99 (0.95–1.01) | 0.96 (0.93–1.00) | 0.111 |
| Total bilirubin (mg/dL) | 0.66 (0.54–0.87) | 0.68 (0.57–0.80) | 0.920 |
| AST (IU/L) | 24 (22–27) | 25 (23–28) | 0.228 |
| ALT (IU/L) | 19 (14–24) | 19 (17–22) | 0.779 |
| Creatinine (mg/dL) | 0.68 (0.57–0.85) | 0.65 (0.56–0.75) | 0.487 |
| RMMZ (n = 39) | SEVO (n = 39) | p-Value | |
|---|---|---|---|
| RMMZ administered rate (μg/kg/h) | 1.17 (0.99–1.52) | 0 | <0.001 * |
| Mean SEVO concentration (vol%) | 0 | 2.1 (1.9–2.2) | <0.001 * |
| Remifentanil administered rate (μg/kg/min) | 0.11 (0.08–0.14) | 0.08 (0.05–0.11) | 0.001 * |
| Inotropic administration (n) | 6 (15.4%) | 26 (66.7%) | <0.001 * |
| Vasodilator administration (n) | 33 (84.6%) | 8 (20.5%) | <0.001 * |
| Sedative administration (n) | 8 (20.5%) | 0 (0.0%) | 0.009 * |
| Anesthesia time (min) | 145 (140–155) | 150 (140–160) | 0.740 |
| Surgical time (min) | 105 (97.5–115) | 105 (95–110) | 0.948 |
| Fluid infusion (mL) | 400 (300–450) | 350 (300–400) | 0.362 |
| Estimated blood loss (mL) | 30 (30–50) | 30 (25–50) | 0.913 |
| RMMZ (n = 39) | SEVO (n = 39) | p-Value | |
|---|---|---|---|
| Emergence time | |||
| Eye open (s) | 225 (162–380) | 372 (312–496) | 0.001 * |
| Extubation (s) | 348 (215–442) | 428 (336–570) | 0.006 * |
| Exit the OR (s) | 392 (252–561) | 479 (386–648) | 0.008 * |
| Aldrete score ≥ 9 (min) | 29.7 (27.0–36.1) | 31.0 (28.6–34.2) | 0.269 |
| Postoperative Cr (mg/dL) | 0.85 (0.74–0.96) | 0.85 (0.78–1.00) | 0.869 |
| Postop Cr—Preop Cr | 0.19 (0.10–0.25) | 0.22 (0.15–0.26) | 0.151 |
| Postoperative HLOS (d) | 8 (6–13) | 7 (6–13) | 0.932 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Kang, H.Y.; Ahn, Y.N.; You, A.H. Comparison of the Incidence of Postoperative Acute Kidney Injury Following the Administration of Remimazolam or Sevoflurane in Elderly Patients Undergoing Total Knee Arthroplasty: A Randomized Controlled Trial. J. Pers. Med. 2023, 13, 789. https://doi.org/10.3390/jpm13050789
Lee S, Kang HY, Ahn YN, You AH. Comparison of the Incidence of Postoperative Acute Kidney Injury Following the Administration of Remimazolam or Sevoflurane in Elderly Patients Undergoing Total Knee Arthroplasty: A Randomized Controlled Trial. Journal of Personalized Medicine. 2023; 13(5):789. https://doi.org/10.3390/jpm13050789
Chicago/Turabian StyleLee, Sangho, Hee Yong Kang, Ye Na Ahn, and Ann Hee You. 2023. "Comparison of the Incidence of Postoperative Acute Kidney Injury Following the Administration of Remimazolam or Sevoflurane in Elderly Patients Undergoing Total Knee Arthroplasty: A Randomized Controlled Trial" Journal of Personalized Medicine 13, no. 5: 789. https://doi.org/10.3390/jpm13050789