Association of HTTLPR, BDNF, and FTO Genetic Variants with Completed Suicide in Slovakia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Genotyping
2.3. Statistical Analysis
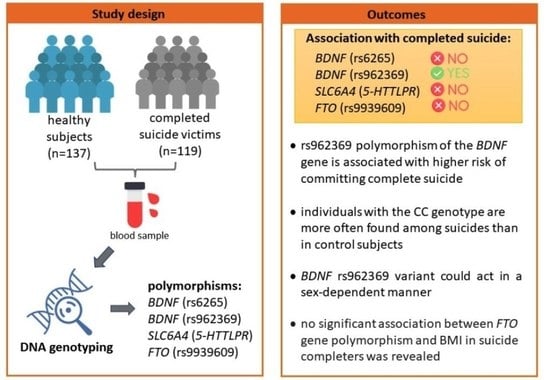
3. Results
3.1. Characterization of the Subjects
3.2. Distribution of Genotypes of Selected Genetic Variants
3.3. Association between HTTLPR, FTO, and BDNF Gene Variants and BMI in Suicide Completers
4. Discussion
4.1. Evaluation of an Association of BDNF Genetic Variants with Suicide
4.2. Evaluation of an Association of 5-HTTLPR (ins/del) Genetic Variants with Suicide
4.3. The FTO Genetic Variant (rs9939609) in the Context of Suicide and BMI
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Suicide. Available online: https://www.who.int/news-room/fact-sheets/detail/suicide (accessed on 30 October 2022).
- Pathirathna, M.L.; Nandasena, H.M.R.K.G.; Atapattu, A.M.M.P.; Weerasekara, I. Impact of the COVID-19 Pandemic on Suicidal Attempts and Death Rates: A Systematic Review. BMC Psychiatry 2022, 22, 1–15. [Google Scholar] [CrossRef]
- Farooq, S.; Tunmore, J.; Ali, W.; Ayub, M. Suicide, Self-Harm and Suicidal Ideation during COVID-19: A Systematic Review. Psychiatry Res. 2021, 306, 114228. [Google Scholar] [CrossRef] [PubMed]
- WHO. Suicide Rates. Available online: https://www.who.int/data/gho/data/themes/mental-health/suicide-rates (accessed on 30 October 2022).
- Brazinova, A.; Moravansky, N.; Gulis, G.; Skodacek, I. Suicide Rate Trends in the Slovak Republic in 1993–2015. Int. J. Soc. Psychiatry 2017, 63, 161–168. [Google Scholar] [CrossRef] [PubMed]
- WHO. Preventing Suicide: A Global Imperative. 2014; pp. 1–89. Available online: https://www.who.int/publications/i/item/9789241564779 (accessed on 7 March 2023).
- Hawton, K.; van Heeringen, K. Suicide. Lancet 2009, 373, 1372–1381. [Google Scholar] [CrossRef]
- Diblasi, E.; Kang, J.; Docherty, A.R. Genetic Contributions to Suicidal Thoughts and Behaviors. Psychol. Med. 2021, 51, 2148–2155. [Google Scholar] [CrossRef]
- Brent, D.A.; Mann, J.J. Family Genetic Studies, Suicide, and Suicidal Behavior. Am. J. Med. Genet. C Semin. Med. Genet. 2005, 133C, 13–24. [Google Scholar] [CrossRef]
- Mirkovic, B.; Laurent, C.; Podlipski, M.A.; Frebourg, T.; Cohen, D.; Gerardin, P. Genetic Association Studies of Suicidal Behavior: A Review of the Past 10 Years, Progress, Limitations, and Future Directions. Front. Psychiatry 2016, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Schenkel, L.C.; Segal, J.; Becker, J.A.; Manfro, G.G.; Bianchin, M.M.; Leistner-Segal, S. The BDNF Val66Met Polymorphism Is an Independent Risk Factor for High Lethality in Suicide Attempts of Depressed Patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 940–944. [Google Scholar] [CrossRef]
- Fusar-Poli, L.; Aguglia, A.; Amerio, A.; Orsolini, L.; Salvi, V.; Serafini, G.; Volpe, U.; Amore, M.; Aguglia, E. Peripheral BDNF Levels in Psychiatric Patients with and without a History of Suicide Attempt: A Systematic Review and Meta-Analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 111, 110342. [Google Scholar] [CrossRef]
- Ropret, S.; Kouter, K.; Zupanc, T.; Paska, A.V. BDNF Methylation and MRNA Expression in Brain and Blood of Completed Suicides in Slovenia. World J. Psychiatry 2021, 11, 1301. [Google Scholar] [CrossRef]
- González-Castro, T.B.; Salas-Magaña, M.; Juárez-Rojop, I.E.; López-Narváez, M.L.; Tovilla-Zárate, C.A.; Hernández-Díaz, Y. Exploring the Association between BDNF Val66Met Polymorphism and Suicidal Behavior: Meta-Analysis and Systematic Review. J. Psychiatr Res. 2017, 94, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Perroud, N.; Aitchison, K.J.; Uher, R.; Smith, R.; Huezo-Diaz, P.; Marusic, A.; Maier, W.; Mors, O.; Placentino, A.; Henigsberg, N.; et al. Genetic Predictors of Increase in Suicidal Ideation During Antidepressant Treatment in the GENDEP Project. Neuropsychopharmacology 2009, 34, 2517–2528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ropret, S.; Zupanc, T.; Komel, R.; Videtič Paska, A. Single Nucleotide Polymorphisms in the BDNF Gene and Suicide in the Slovenian Sample. Neurosci. Lett. 2015, 602, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Bednarova, A.; Cizmarikova, M.; Habalova, V.; Jarcuskova, D. Evaluation of 5-HTTLPR (Insertion/Deletion) and BDNF (Rs6265) Genetic Variations in the Slovakian Individuals Suffering from Affective Disorders. Gen. Physiol. Biophys. 2021, 40, 365–376. [Google Scholar] [CrossRef]
- Fanelli, G.; Serretti, A. The Influence of the Serotonin Transporter Gene 5-HTTLPR Polymorphism on Suicidal Behaviors: A Meta-Analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 88, 375–387. [Google Scholar] [CrossRef]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.B.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A Common Variant in the FTO Gene Is Associated with Body Mass Index and Predisposes to Childhood and Adult Obesity. Science 2007, 316, 889. [Google Scholar] [CrossRef] [Green Version]
- Dina, C.; Meyre, D.; Gallina, S.; Durand, E.; Körner, A.; Jacobson, P.; Carlsson, L.M.S.; Kiess, W.; Vatin, V.; Lecoeur, C.; et al. Variation in FTO Contributes to Childhood Obesity and Severe Adult Obesity. Nat. Genet. 2007, 39, 724–726. [Google Scholar] [CrossRef]
- Yao, Y.; Wen, Y.; Du, T.; Sun, N.; Deng, H.; Ryan, J.; Rao, S. Meta-Analysis Indicates That SNP Rs9939609 within FTO Is Not Associated with Major Depressive Disorder (MDD) in Asian Population. J. Affect. Disord. 2016, 193, 27–30. [Google Scholar] [CrossRef]
- Du, T.; Rao, S.; Wu, L.; Ye, N.; Liu, Z.; Hu, H.; Xiu, J.; Shen, Y.; Xu, Q. An Association Study of the M6A Genes with Major Depressive Disorder in Chinese Han Population. Artic. J. Affect. Disord. 2015, 183, 279–286. [Google Scholar] [CrossRef]
- Chojnicka, I.; Fudalej, S.; Walczak, A.; Wasilewska, K.; Fudalej, M.; Stawinski, P.; Strawa, K.; Pawlak, A.; Wojnar, M.; Krajewski, P.; et al. Inverse Association between Obesity Predisposing FTO Genotype and Completed Suicide. PLoS ONE 2014, 9, e108900. [Google Scholar] [CrossRef]
- Li, L.; Zang, L.; Zhang, F.; Chen, J.; Shen, H.; Shu, L.; Liang, F.; Feng, C.; Chen, D.; Tao, H.; et al. Fat Mass and Obesity-Associated (FTO) Protein Regulates Adult Neurogenesis. Hum. Mol. Genet. 2017, 26, 2398–2411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, M.E.; Hess, S.; Meyer, K.D.; Verhagen, L.A.W.; Koch, L.; Brönneke, H.S.; Dietrich, M.O.; Jordan, S.D.; Saletore, Y.; Elemento, O.; et al. The Fat Mass and Obesity Associated Gene (Fto) Regulates Activity of the Dopaminergic Midbrain Circuitry. Nat. Neurosci. 2013, 16, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Widagdo, J.; Zhao, Q.Y.; Kempen, M.J.; Tan, M.C.; Ratnu, V.S.; Wei, W.; Leighton, L.; Spadaro, P.A.; Edson, J.; Anggono, V.; et al. Experience-Dependent Accumulation of N6-Methyladenosine in the Prefrontal Cortex Is Associated with Memory Processes in Mice. J. Neurosci. 2016, 36, 6771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spychala, A.; Rüther, U. FTO Affects Hippocampal Function by Regulation of BDNF Processing. PLoS ONE 2019, 14, e0211937. [Google Scholar] [CrossRef] [PubMed]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut Off Points; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Murakami, F.; Shimomura, T.; Kotani, K.; Ikawa, S.; Nanba, E.; Adachi, K. Anxiety Traits Associated with a Polymorphism in the Serotonin Transporter Gene Regulatory Region in the Japanese. J. Hum. Genet. 1999, 44, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Solé, X.; Guinó, E.; Valls, J.; Iniesta, R.; Moreno, V. SNPStats: A Web Tool for the Analysis of Association Studies. Bioinformatics 2006, 22, 1928–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabrinskas, R.; Hamilton, B.; Daniel, C.; Oliffe, J. Suicide by Hanging: A Scoping Review. Int. J. Ment. Health Nurs. 2022, 31, 278–294. [Google Scholar] [CrossRef]
- Gunnell, D.; Bennewith, O.; Hawton, K.; Simkin, S.; Kapur, N. The Epidemiology and Prevention of Suicide by Hanging: A Systematic Review. Int. J. Epidemiol. 2005, 34, 433–442. [Google Scholar] [CrossRef] [Green Version]
- Chojnicka, I.; Strawa, K.; Fudalej, S.; Fudalej, M.; Pawlak, A.; Kostrzewa, G.; Wojnar, M.; Krajewski, P.; Poski, R. Analysis of Four Genes Involved in the Neurodevelopment Shows Association of Rs4307059 Polymorphism in the Cadherin 9/10 Region with Completed Suicide. Neuropsychobiology 2012, 66, 134–140. [Google Scholar] [CrossRef]
- Nedic, G.; Nikolac Perkovic, M.; Nenadic Sviglin, K.; Muck-Seler, D.; Borovecki, F.; Pivac, N. Brain-Derived Neurotrophic Factor Val66Met Polymorphism and Alcohol-Related Phenotypes. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 40, 193–198. [Google Scholar] [CrossRef]
- Zarrilli, F.; Angiolillo, A.; Castaldo, G.; Chiariotti, L.; Keller, S.; Sacchetti, S.; Marusic, A.; Zagar, T.; Carli, V.; Roy, A.; et al. Brain Derived Neurotrophic Factor (BDNF) Genetic Polymorphism (Val66Met) in Suicide: A Study of 512 Cases. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2009, 150B, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Pregelj, P.; Nedic, G.; Paska, A.V.; Zupanc, T.; Nikolac, M.; Balažic, J.; Tomori, M.; Komel, R.; Seler, D.M.; Pivac, N. The Association between Brain-Derived Neurotrophic Factor Polymorphism (BDNF Val66Met) and Suicide. J. Affect. Disord. 2011, 128, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Berent, D.; Szymańska, B.; Kulczycka-Wojdala, D.; Macander, M.; Pawłowska, Z.; Wojnar, M. The Role of Childhood Adversities, FKBP5, BDNF, NRN1, and Generalized Self-Efficacy in Suicide Attempts in Alcohol-Dependent Patients. Pharmacol. Rep. 2020, 72, 730. [Google Scholar] [CrossRef] [PubMed]
- Schosser, A.; Carlberg, L.; Calati, R.; Serretti, A.; Massat, I.; Spindelegger, C.; Linotte, S.; Mendlewicz, J.; Souery, D.; Zohar, J.; et al. The Impact of BDNF Polymorphisms on Suicidality in Treatment-Resistant Major Depressive Disorder: A European Multicenter Study. Int. J. Neuropsychopharmacol. 2017, 20, 782. [Google Scholar] [CrossRef] [Green Version]
- Monteggia, L.M.; Luikart, B.; Barrot, M.; Theobold, D.; Malkovska, I.; Nef, S.; Parada, L.F.; Nestler, E.J. Brain-Derived Neurotrophic Factor Conditional Knockouts Show Gender Differences in Depression-Related Behaviors. Biol. Psychiatry 2007, 61, 187–197. [Google Scholar] [CrossRef]
- Fanaei, H.; Karimian, S.M.; Sadeghipour, H.R.; Hassanzade, G.; Kasaeian, A.; Attari, F.; Khayat, S.; Ramezani, V.; Javadimehr, M. Testosterone Enhances Functional Recovery after Stroke through Promotion of Antioxidant Defenses, BDNF Levels and Neurogenesis in Male Rats. Brain Res. 2014, 1558, 74–83. [Google Scholar] [CrossRef]
- Li, M.; Masugi-Tokita, M.; Takanami, K.; Yamada, S.; Kawata, M. Testosterone Has Sublayer-Specific Effects on Dendritic Spine Maturation Mediated by BDNF and PSD-95 in Pyramidal Neurons in the Hippocampus CA1 Area. Brain Res. 2012, 1484, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Anguelova, M.; Benkelfat, C.; Turecki, G. A Systematic Review of Association Studies Investigating Genes Coding for Serotonin Receptors and the Serotonin Transporter: II. Suicidal Behavior. Mol. Psychiatry 2003, 8, 646–653. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; He, L. Meta-Analysis Supports Association between Serotonin Transporter (5-HTT) and Suicidal Behavior. Mol. Psychiatry 2007, 12, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Schild, A.H.E.; Pietschnig, J.; Tran, U.S.; Voracek, M. Genetic Association Studies between SNPs and Suicidal Behavior: A Meta-Analytical Field Synopsis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 46, 36–42. [Google Scholar] [CrossRef]
- Clayden, R.C.; Zaruk, A.; Meyre, D.; Thabane, L.; Samaan, Z. The Association of Attempted Suicide with Genetic Variants in the SLC6A4 and TPH Genes Depends on the Definition of Suicidal Behavior: A Systematic Review and Meta-Analysis. Transl. Psychiatry 2012, 2, e166. [Google Scholar] [CrossRef] [Green Version]
- De Medeiros Alves, V.; Bezerra, D.; de Andrade, T.; de Melo Neto, V.; Nardi, A. Genetic Polymorphisms Might Predict Suicide Attempts in Mental Disorder Patients: A Systematic Review and Meta-Analysis. CNS Neurol. Disord. Drug Targets 2015, 14, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Tsai, G. Association between Serotonin Transporter Gene Promoter Polymorphism and Suicide: Results of a Meta-Analysis. Biol. Psychiatry 2004, 55, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Bokor, J.; Gonda, X.; Dome, P.; Faludi, G.; Dinya, E.; Laszik, A. Association between the 5-HTTLPR Polymorphism of the Serotonin Transporter Gene and Suicide: A Case-Control Pilot Study. Neuropsychopharmacol. Hung. 2017, 19, 5–10. [Google Scholar] [PubMed]
- Pawlak, J.; Dmitrzak-Weglarz, M.; Wilkosc, M.; Szczepankiewicz, A.; Leszczynska-Rodziewicz, A.; Zaremba, D.; Kapelski, P.; Rajewska-Rager, A.; Hauser, J. Suicide Behavior as a Quantitative Trait and Its Genetic Background. J. Affect. Disord. 2016, 206, 241–250. [Google Scholar] [CrossRef]
- Božina, N.; Jovanović, N.; Podlesek, A.; Rojnić Kuzman, M.; Kudumija Slijepčević, M.; Roguljić, A.; Dimitrović, A.; Božina, T.; Lovrić, J.; Ljubić, H.; et al. Suicide Ideators and Attempters with Schizophrenia—The Role of 5-HTTLPR, Rs25531, and 5-HTT VNTR Intron 2 Variants. J. Psychiatr. Res. 2012, 46, 767–773. [Google Scholar] [CrossRef] [Green Version]
- Hranilovic, D.; Stefulj, J.; Furac, I.; Kubat, M.; Balija, M.; Jernej, B. Serotonin Transporter Gene Promoter (5-HTTLPR) and Intron 2 (VNTR) Polymorphisms in Croatian Suicide Victims. Biol. Psychiatry 2003, 54, 884–889. [Google Scholar] [CrossRef]
- Pungercic, G.; Videtic, A.; Pestotnik, A.; Pajnic, I.Z.; Zupanc, T.; Balazic, J.; Tomori, M.; Komel, R. Serotonin Transporter Gene Promoter (5-HTTLPR) and Intron 2 (VNTR) Polymorphisms: A Study on Slovenian Population of Suicide Victims. Psychiatr. Genet. 2006, 16, 187–191. [Google Scholar] [CrossRef]
- Schild, A.H.E.; Nader, I.W.; Pietschnig, J.; Voracek, M. Ethnicity Moderates the Association Between 5-HTTLPR and National Suicide Rates. Arch. Suicide Res. 2014, 18, 1–13. [Google Scholar] [CrossRef]
- Limosin, F.; Loze, J.Y.; Boni, C.; Hamon, M.; Adès, J.; Rouillon, F.; Gorwood, P. Male-Specific Association between the 5-HTTLPR S Allele and Suicide Attempts in Alcohol-Dependent Subjects. J. Psychiatr. Res. 2005, 39, 179–182. [Google Scholar] [CrossRef]
- Gaysina, D.; Zainullina, A.; Gabdulhakov, R.; Khusnutdinova, E. The Serotonin Transporter Gene: Polymorphism and Haplotype Analysis in Russian Suicide Attempters. Neuropsychobiology 2006, 54, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, A.; Sanna, S.; Chen, W.M.; Uda, M.; Albai, G.; Strait, J.; Najjar, S.; Nagaraja, R.; Orrú, M.; Usala, G.; et al. Genome-Wide Association Scan Shows Genetic Variants in the FTO Gene Are Associated with Obesity-Related Traits. PLoS Genet. 2007, 3, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.J.H.; Zitman, F.G. Overweight, Obesity, and Depression: A Systematic Review and Meta-Analysis of Longitudinal Studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Zarza-Rebollo, J.A.; Molina, E.; Rivera, M. The Role of the FTO Gene in the Relationship between Depression and Obesity. A Systematic Review. Neurosci. Biobehav. Rev. 2021, 127, 630–637. [Google Scholar] [CrossRef]
- Wray, N.R.; Ripke, S.; Mattheisen, M.; Trzaskowski, M.; Byrne, E.M.; Abdellaoui, A.; Adams, M.J.; Agerbo, E.; Air, T.M.; Andlauer, T.M.F.; et al. Genome-Wide Association Analyses Identify 44 Risk Variants and Refine the Genetic Architecture of Major Depression. Nat. Genet. 2018, 50, 668–681. [Google Scholar] [CrossRef] [Green Version]
- Milaneschi, Y.; Lamers, F.; Berk, M.; Penninx, B.W.J.H. Depression Heterogeneity and Its Biological Underpinnings: Toward Immunometabolic Depression. Biol. Psychiatry 2020, 88, 369–380. [Google Scholar] [CrossRef]
- Rivera, M.; Locke, A.E.; Corre, T.; Czamara, D.; Wolf, C.; Ching-Lopez, A.; Milaneschi, Y.; Kloiber, S.; Cohen-Woods, S.; Rucker, J.; et al. Interaction between the FTO Gene, Body Mass Index and Depression: Meta-Analysis of 13701 Individuals. Br. J. Psychiatry 2017, 211, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Samaan, Z.; Anand, S.; Zhang, X.; Desai, D.; Rivera, M.; Pare, G.; Thabane, L.; Xie, C.; Gerstein, H.; Engert, J.C.; et al. The Protective Effect of the Obesity-Associated Rs9939609 A Variant in Fat Mass- and Obesity-Associated Gene on Depression. Mol. Psychiatry 2013, 18, 1281–1286. [Google Scholar] [CrossRef]
| Gene | Oligonucleotides | Sequences |
|---|---|---|
| FTO rs9939609 | forward-limit | 5′-GCATTTAGAATGTCTGAATTATTATTCTAG-3′ |
| reverse-excess | 5′-CCTATTAAAACTTTAGAGTAACAGAG-3′ | |
| probe | 5′-TGCTGTGAATTTTGTGATGCACTTGGAT-Phos′ | |
| BDNF rs6265 | forward-limit | 5′-GCCGAACTTTCTGGTCCTCATCC-3′ |
| reverse-excess | 5′-AAGGCAGGTTCAAGAGGCTTG-3 | |
| probe | 5′-GCTCTTCTATCACGTGTTCGAAAGTGTC-Phos | |
| BDNF rs962369 | forward-limit | 5′-GACATTTTTATGAGAAGGGTTTACATAAG-3′ |
| reverse-excess | 5′-AAAGAATTGCTCACTGTAATGAC-3′ | |
| probe | 5′-TGCCAAGAGAGTTGAGTCCATGG-Phos |
| Age | Control | Suicides | ||||
|---|---|---|---|---|---|---|
| Total (n = 137) | Males (n = 107) | Females (n = 30) | Total (n = 119) | Males (n = 92) | Females (n = 27) | |
| average ± SD | 48.23 ± 18.85 | 47.36 ± 18.75 | 51.33 ± 18.86 | 47.71 ± 17.57 | 46.86 ± 16.65 | 50.63 ± 20.11 |
| median | 47 | 45 | 52.5 | 48 | 46.5 | 51 |
| min–max | 18–91 | 21–90 | 18–91 | 14–90 | 14–85 | 15–90 |
| <35 years | 40 (29.20%) | 34 (31.78%) | 6 (20.00%) | 31 (26.05%) | 26 (28.26%) | 5 (18.52%) |
| 35–49 years | 30 (21.90%) | 25 (23.36%) | 5 (16.67%) | 35 (29.41%) | 29 (31.52%) | 6 (22.22%) |
| >50 years | 67 (48.91%) | 48 (44.86%) | 19 (63.33%) | 53 (44.54%) | 37 (40.22%) | 16 (59.26%) |
| Total | Males | Females | ||||
|---|---|---|---|---|---|---|
| Method of Suicide | n | % | n | % | n | % |
| hanging or suffocation | 71 | 59.6% | 58 | 61.1% | 13 | 48.2% |
| jumping from high places | 16 | 13.4% | 9 | 9.47% | 7 | 25.9% |
| collision with a train | 12 | 10.1% | 7 | 7.37% | 5 | 18.5% |
| shooting | 11 | 9.24% | 11 | 11.6% | 0 | 0.00% |
| cutting | 5 | 4.20% | 5 | 5.26% | 0 | 0.00% |
| electrocution | 2 | 1.68% | 2 | 2.11% | 0 | 0.00% |
| poisoning | 2 | 1.68% | 0 | 0.00% | 2 | 7.41% |
| BMI | ||||||
| average ± SD | 24.22 ± 3.83 | 24.17 ± 3.30 | 24.42 ± 5.35 | |||
| median | 23.84 | 23.86 | 23.53 | |||
| min–max | 13.6–35.7 | 13.9–33.31 | 13.67–35.7 | |||
| BMI < 18.5 (underweight) | 6 | 5.04% | 3 | 3.26% | 3 | 11.1% |
| BMI = 18.5–24.9 (normal) | 71 | 59.6% | 61 | 66.3% | 10 | 37.4% |
| BMI = 25–29.9 (overweight) | 3 | 27.7% | 22 | 23.9% | 11 | 40.7% |
| BMI > 30 (obese) | 9 | 7.56% | 6 | 6.52% | 3 | 11.1% |
| Subjects | Genotype/Allele | Control n (%) | Suicides n (%) | Odds Ratio (95% CI) | p-Value | HWE Controls |
|---|---|---|---|---|---|---|
| 5-HTTLPR (ins/del) | ||||||
| Codominant model | ||||||
| Total | LL | 32 (23.4%) | 19 (16.0%) | 1.00 | 0.32 | 0.94 |
| LS | 68 (49.6%) | 63 (52.9%) | 1.56 (0.80–3.03) | |||
| SS | 37 (27.0%) | 37 (31.1%) | 1.68 (0.81–3.49) | |||
| Allele, S (proportion) | 142 (0.52) | 137 (0.58) | ||||
| Males | LL | 25 (23.4%) | 17 (18.5%) | 1.00 | 0.65 | 0.53 |
| LS | 50 (46.7%) | 48 (52.2%) | 1.41 (0.68–2.94) | |||
| SS | 32 (29.9%) | 27 (29.4%) | 1.24 (0.56–2.77) | |||
| Allele, S (proportion) | 114 (0.53) | 102 (0.55) | ||||
| Females | LL | 7 (23.3%) | 2 (7.4%) | 1.00 | 0.09 | 0.26 |
| LS | 18 (60.0%) | 15 (55.6%) | 2.92 (0.53–16.19) | |||
| SS | 5 (16.7%) | 10 (37.0%) | 7.00 (1.04–46.94) | |||
| Allele, S (proportion) | 28 (0.47) | 35 (0.65) | ||||
| FTO (rs9939609) | ||||||
| Codominant model | ||||||
| Total | TT | 42 (30.7%) | 46 (38.7%) | 1.00 | 0.40 | 0.61 |
| TA | 65 (47.5%) | 49 (41.2%) | 0.69 (0.39–1.20) | |||
| AA | 30 (21.9%) | 24 (20.2%) | 0.73 (0.37–1.44) | |||
| Allele, A (proportion) | 125 (0.46) | 97 (0.41) | ||||
| Males | TT | 34 (31.8%) | 36 (39.1%) | 1.00 | 0.55 | 0.89 |
| TA | 52 (48.6%) | 39 (42.4%) | 0.71 (0.38–1.32) | |||
| AA | 21 (19.6%) | 17 (18.5%) | 0.76 (0.35–1.69) | |||
| Allele, A (proportion) | 94 (0.44) | 73 (0.40) | ||||
| Females | TT | 8 (26.7%) | 10 (37.0%) | 1.00 | 0.70 | 0.47 |
| TA | 13 (43.3%) | 10 (37.0%) | 0.62 (0.18–2.13) | |||
| AA | 9 (30%) | 7 (26.0%) | 0.62 (0.16–2.42) | |||
| Allele, A (proportion) | 31 (0.52) | 24 (0.44) | ||||
| Subjects | Genotype/Allele | Control n (%) | Suicides n (%) | Odds Ratio (95% CI) | p-Value | HWE Controls |
|---|---|---|---|---|---|---|
| BDNF (rs6265) | ||||||
| Codominant model | ||||||
| Total | CC | 92 (67.2%) | 82 (68.9%) | 1.00 | 0.92 | 0.82 |
| CT | 41 (29.9%) | 33 (27.7%) | 0.90 (0.52–1.56) | |||
| TT | 4 (2.9%) | 4 (3.4%) | 1.12 (0.27–4.63) | |||
| Allele, T (proportion) | 49 (18%) | 41 (17%) | ||||
| Males | CC | 75 (70.1%) | 63 (68.5%) | 1.00 | 0.84 | 0.92 |
| CT | 29 (27.1%) | 25 (27.2%) | 1.03 (0.55–1.93) | |||
| TT | 3 (2.8%) | 4 (4.3%) | 1.59 (0.34–7.36) | |||
| Allele, T (proportion) | 35 (0.16) | 33 (0.18) | ||||
| Females | CC | 17 (56.7%) | 19 (70.4%) | 1.00 | 0.34 | 0.52 |
| CT | 12 (40.0%) | 8 (29.6%) | 0.60 (0.20–1.81) | |||
| TT | 1 (3.3%) | 0 (0.0%) | 0.00 (0.00–NA) | |||
| Allele, T (proportion) | 14 (0.23) | 8 (0.15) | ||||
| BDNF (rs962369) | ||||||
| Codominant model | ||||||
| Total | TT | 81 (59.1%) | 67 (56.3%) | 1.00 | 0.09 | 0.20 |
| TC | 52 (38.0%) | 41 (34.5%) | 0.95 (0.56–1.60) | |||
| CC | 4 (2.9%) | 11 (9.2%) | 3.32 (1.01–10.92) | |||
| Allele, C (proportion) | 60 (0.22) | 63 (0.26) | ||||
| Males | TT | 65 (60.8%) | 51 (55.4%) | 1.00 | 0.04 | |
| TC | 39 (36.5%) | 30 (32.6%) | 0.98 (0.54–1.79) | |||
| CC | 3 (2.8%) | 11 (12.0%) | 4.67 (1.24–17.64) | |||
| Allele, C (proportion) | 97 (0.24) | 45 (0.21) | 52 (0.28) | |||
| Females | TT | 16 (53.3%) | 16 (59.3%) | 1.00 | 0.50 | 0.39 |
| TC | 13 (43.3%) | 11 (40.7%) | 0.85 (0.29–2.44) | |||
| CC | 1 (3.3%) | 0 (0.0%) | 0.00 (0.00-NA) | |||
| Allele, C (proportion) | 15 (0.25) | 11 (0.2) | ||||
| Recessive model | ||||||
| Total | TT+TC | 133 (97.1%) | 108 (90.8%) | 1.00 | 0.03 | |
| CC | 4 (2.9%) | 11 (9.2%) | 3.39 (1.05–10.94) | |||
| Males | TT+TC | 104 (97.2%) | 81 (88.0%) | 1.00 | 0.01 | |
| CC | 3 (2.8%) | 11 (12.0%) | 4.71 (1.27–17.43) | |||
| Gene | Model | Gender | Genotype | BMI mean (s.e.) | Difference (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| HTTLPR | ||||||
| ins/del | Codominant | Total | LL | 23.93 (0.67) | ref. | 0.90 |
| LS | 24.28 (0.47) | 0.35 (−1.20–1.90) | ||||
| SS | 24.05 (0.82) | 0.12 (−1.99–2.24) | ||||
| Male | LL | 24.10 (0.50) | ref. | 1.00 | ||
| LS | 24.03 (0.51) | −0.07 (−1.64–1.50) | ||||
| SS | 24.06 (0.90) | −0.04 (−2.06–1.98) | ||||
| Female | LL | 23.47 (2.14) | ref. | 0.76 | ||
| LS | 25.08 (1.13) | 1.61 (−2.68–5.90) | ||||
| SS | 23.96 (2.16) | 0.49 (−7.65–8.63) | ||||
| FTO | ||||||
| rs9939609 | Codominant | Total | TT | 23.88 (0.46) | ref. | 0.65 |
| TA | 24.53 (0.62) | 0.65 (−0.88–2.18) | ||||
| AA | 23.84 (0.80) | −0.03 (−1.91–1.85) | ||||
| Male | TT | 24.20 (0.44) | ref. | 0.71 | ||
| TA | 24.19 (0.61) | −0.02 (−1.52–1.49) | ||||
| AA | 23.46 (0.85) | −0.74 (−2.66–1.17) | ||||
| Female | TT | 22.69 (1.43) | ref. | 0.41 | ||
| TA | 25.85 (1.90) | 3.15 (−1.43–7.73) | ||||
| AA | 24.78 (1.86) | 2.08 (−2.96–7.13) | ||||
| BDNF | ||||||
| rs6265 | Codominant | Total | CC | 24.30 (0.40) | ref. | 0.55 |
| CT | 23.59 (0.74) | −0.71 (−2.25–0.83) | ||||
| TT | 25.27 (2.04) | 0.96 (−2.85–4.78) | ||||
| Male | CC | 24.05 (0.41) | ref. | 0.74 | ||
| CT | 23.88 (0.68) | −0.17 (−1.71–1.37) | ||||
| TT | 25.27 (2.04) | 1.21 (−2.14–4.57) | ||||
| Female | CC | 25.13 (1.07) | ref. | 0.27 | ||
| CT | 22.68 (2.24) | −2.45 (−6.73–1.83) | ||||
| TT | NA | - | ||||
| BDNF | ||||||
| rs962369 | Codominant | Total | TT | 24.65 (0.43) | ref. | 0.20 |
| TC | 23.30 (0.66) | −1.35 (−2.81–0.12) | ||||
| CC | 24.14 (1.04) | −0.51 (−2.92–1.89) | ||||
| Male | TT | 24.71 (0.46) | ref. | 0.06 | ||
| TC | 22.92 (0.57) | −1.79 (−3.25–−0.33) | ||||
| CC | 24.14 (1.04) | −0.57 (−2.68–1.53) | ||||
| Female | TT | 24.45 (1.07) | ref. | 0.96 | ||
| TC | 24.34 (1.98) | −0.11 (−4.19–3.97) | ||||
| CC | NA | - | ||||
| Overdominant | Male | TT and CC | 24.61 (0.42) | ref. | 0.02 | |
| TC | 22.92 (0.57) | −1.69 (−3.09–−0.29) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednarova, A.; Habalova, V.; Iannaccone, S.F.; Tkac, I.; Jarcuskova, D.; Krivosova, M.; Marcatili, M.; Hlavacova, N. Association of HTTLPR, BDNF, and FTO Genetic Variants with Completed Suicide in Slovakia. J. Pers. Med. 2023, 13, 501. https://doi.org/10.3390/jpm13030501
Bednarova A, Habalova V, Iannaccone SF, Tkac I, Jarcuskova D, Krivosova M, Marcatili M, Hlavacova N. Association of HTTLPR, BDNF, and FTO Genetic Variants with Completed Suicide in Slovakia. Journal of Personalized Medicine. 2023; 13(3):501. https://doi.org/10.3390/jpm13030501
Chicago/Turabian StyleBednarova, Aneta, Viera Habalova, Silvia Farkasova Iannaccone, Ivan Tkac, Dominika Jarcuskova, Michaela Krivosova, Matteo Marcatili, and Natasa Hlavacova. 2023. "Association of HTTLPR, BDNF, and FTO Genetic Variants with Completed Suicide in Slovakia" Journal of Personalized Medicine 13, no. 3: 501. https://doi.org/10.3390/jpm13030501