Radiosurgery and Stereotactic Brain Radiotherapy with Systemic Therapy in Recurrent High-Grade Gliomas: Is It Feasible? Therapeutic Strategies in Recurrent High-Grade Gliomas
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
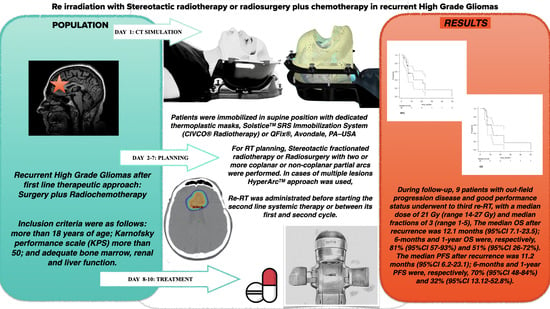
3. Results
Prognostic Factors for OS and PFS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Ward, E.; Murray, T.; Xu, J.; Smigal, C.; Thun, M.J. Cancer statistics. CA Cancer J Clin. 2006, 56, 106–130. [Google Scholar] [CrossRef] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; Van Den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Yazici, G.; Cengiz, M.; Özyigit, G.; Eren, G.; Yildiz, F.; Akyol, F.; Gurkaynak, M.; Zorlu, F. Hypofractionated stereotactic reirradiation for recurrent glioblastoma. J. Neurooncol. 2014, 120, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carrillo, M.; Tovar-Marítn, I.; Zurita-Herrera, M.; Del Moral-Ávila, R.; Guerrero-Tejada, R.; Saura-Rojas, E.; Osorio-Ceballos, J.L.; Arrebola-Moreno, J.P.; Expósito-Hernández, J. Salvage radiosurgery for selected patients with recurrent malignant gliomas. BioMed Res. Int. 2014, 2014, 657953. [Google Scholar] [CrossRef] [PubMed]
- Biswas, T.; Okunieff, P.; Schell, M.C.; Smudzin, T.; Pilcher, W.H.; Bakos, R.S.; Vates, G.E.; Walter, K.A.; Wensel, A.; Korones, D.N.; et al. Stereotactic radiosurgery for glioblastoma: Retrospective analysis. Radiat. Oncol. 2009, 4, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niranjan, A.; Kano, H.; Iyer, A.; Kondziolka, D.; Flickinger, J.C.; Lunsford, L.D. Role of adjuvant or salvage radiosurgery in the management of unresected residual or progressive glioblastoma multiforme in the pre-bevacizumab era. J. Neurosurg. 2015, 122, 757–765. [Google Scholar] [CrossRef] [Green Version]
- Park, K.-J.; Kano, H.; Iyer, A.; Liu, X.; Niranjan, A.; Flickinger, J.C.; Lieberman, F.S.; Lunsford, L.D.; Kondziolka, D. Salvage gamma knife stereotactic radiosurgery followed by bevacizumab for recurrent glioblastoma multiforme: A case–control study. J. Neurooncol. 2012, 107, 323–333. [Google Scholar] [CrossRef]
- Patel, M.; Siddiqui, F.; Jin, J.Y.; Mikkelsen, T.; Rosenblum, M.; Movsas, B.; Ryu, S. Salvage reirradiation for recurrent glioblastoma with radiosurgery: Radiographic response and improved survival. J. Neurooncol. 2009, 92, 185–191. [Google Scholar] [CrossRef]
- Skeie, B.S.; Enger, P.Ø.; Brøgger, J.; Ganz, J.C.; Thorsen, F.; Heggdal, J.I.; Pedersen, P.H. γ knife surgery versus reoperation for recurrent glioblastoma multiforme. World Neurosurg. 2012, 78, 658–669. [Google Scholar] [CrossRef]
- Bräutigam, E.; Lampl, C.; Track, C.; Nieder, C.; Pichler, J.; Hammer, J.; Geinitz, H. Re-irradiation of recurrent glioblastoma as part of a sequential multimodality treatment concept. Clin Transl Oncol. 2019, 21, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Frischer, J.M.; Marosi, C.; Woehrer, A.; Hainfellner, J.A.; Dieckmann, K.U.; Eiter, H.; Wang, W.T.; Mallouhi, A.; Ertl, A.; Knosp, E.; et al. Gamma knife radiosurgery in recurrent glioblastoma. Stereotact. Funct. Neurosurg. 2016, 94, 265–272. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Central Nervous System Cancers (Version 2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/cns_blocks.pdf. (accessed on 1 August 2022).
- Bloch, O.; Han, S.J.; Cha, S.; Sun, M.Z.; Aghi, M.K.; McDermott, M.W.; Berger, M.S.; Parsa, A.T. Impact of extent of resection for recurrent glioblastoma on overall survival: Clinical article. J. Neurosurg. 2012, 117, 1032–1038. [Google Scholar] [CrossRef]
- Friedman, H.S.; Prados, M.D.; Wen, P.Y.; Mikkelsen, T.; Schiff, D.; Abrey, L.E.; Yung, W.A.; Paleologos, N.; Nicholas, M.K.; Jensen, R.; et al. Bevacizumab Alone and in Combination with Irinotecan in Recurrent Glioblastoma. J. Clin. Oncol. 2009, 27, 4733–4740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombardi, G.; De Salvo, G.L.; Brandes, A.A.; Eoli, M.; Rudà, R.; Faedi, M.; Lolli, I.; Pace, A.; Daniele, B.; Pasqualetti, F.; et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2019, 20, 110–119. [Google Scholar] [CrossRef]
- Navarria, P.; Minniti, G.; Clerici, E.; Tomatis, S.; Pinzi, V.; Ciammella, P.; Galaverni, M.; Amelio, D.; Scartoni, D.; Scoccianti, S.; et al. Re-irradiation for recurrent glioma: Outcome evaluation, toxicity and prognostic factors assessment. A multicenter study of the Radiation Oncology Italian Association (AIRO). J. Neurooncol. 2019, 142, 59–67. [Google Scholar] [CrossRef]
- Mazzola, R.; Corradini, S.; Gregucci, F.; Figlia, V.; Fiorentino, A.; Alongi, F. Role of Radiosurgery/Stereotactic Radiotherapy in Oligometastatic Disease: Brain Oligometastases. Front. Oncol. 2019, 9, 206. [Google Scholar] [CrossRef] [PubMed]
- Meduri, B.; Gregucci, F.; D’Angelo, E.; Alitto, A.R.; Ciurlia, E.; Desideri, I.; Marino, L.; Borghetti, P.; Fiore, M.; Fiorentino, A. AIRO Giovani-Italian association of radiation oncology-young members. volume de-escalation in radiation therapy: State of the art and new perspectives. J. Cancer Res. Clin. Oncol. 2020, 146, 909–924. [Google Scholar] [CrossRef]
- Fogh, S.E.; Andrews, D.W.; Glass, J.; Curran, W.; Glass, C.; Champ, C.; Evans, J.J.; Hyslop, T.; Pequignot, E.; Downes, B.; et al. Hypofractionated Stereotactic Radiation Therapy: An Effective Therapy for Recurrent High-Grade Gliomas. J. Clin. Oncol. 2010, 28, 3048–3053. [Google Scholar] [CrossRef]
- Holt, D.E.; Bernard, M.E.; Quan, K.; Clump, D.A.; Engh, J.A.; Burton, S.A.; Heron, D.E. Salvage stereotactic radiosurgery for recurrent glioblastoma multiforme with prior radiation therapy. J. Cancer Res. Ther. 2016, 12, 1243–1248. [Google Scholar]
- Martínez-Garcia, M.; Álvarez-Linera, J.; Carrato, C.; Ley, L.; Luque, R.; Maldonado, X.; Martínez-Aguillo, M.; Navarro, L.M.; Vaz-Salgado, M.A.; Gil-Gil, M. SEOM clinical guidelines for diagnosis and treatment of glioblastoma (2017). Clin. Transl. Oncol. 2018, 20, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Torok, J.A.; Wegner, R.E.; Mintz, A.H.; Heron, D.E.; Burton, S.A. Re-irradiation with radiosurgery for recurrent glioblastoma multiforme. Technol. Cancer Res. Treat. 2011, 10, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Gigliotti, M.J.; Hasan, S.; Karlovits, S.M.; Ranjan, T.; Wegner, R.E. Re-irradiation with stereotactic radiosurgery/radiotherapy for recurrent high-grade gliomas: Improved survival in the modern era. Stereotact. Funct. Neurosurg. 2018, 96, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Alongi, F.; Fiorentino, A.; Gregucci, F.; Corradini, S.; Giaj-Levra, N.; Romano, L.; Rigo, M.; Ricchetti, F.; Beltramello, A.; Lunardi, G.; et al. First Experience and Clinical Results Using a New Non-Coplanar Mono-Isocenter Technique (HyperArcTM) for Linac-Based VMAT Radiosurgery in Brain Metastases. J. Cancer Res. Clin. Oncol. 2019, 145, 193–200. [Google Scholar] [CrossRef]
- Gregucci, F.; Bonaparte, I.; Surgo, A.; Caliandro, M.; Carbonara, R.; Ciliberti, M.P.; Aga, A.; Berloco, F.; De Masi, M.; De Pascali, C.; et al. Brain Linac-based Radiation Therapy: “Test Drive” of New Immobilization Solution and Surface Guided Radiation Therapy. J. Pers. Med. 2021, 11, 1351. [Google Scholar] [CrossRef] [PubMed]
- Gregucci, F.; Surgo, A.; Bonaparte, I.; Laera, L.; Ciliberti, M.P.; Carbonara, R.; Gentile, M.A.; Giraldi, D.; Calbi, R.; Caliandro, M.; et al. Poor-Prognosis Patients Affected by Glioblastoma: Retrospective Study of Hypofractionated Radiotherapy with Simultaneous Integrated Boost and Concurrent/Adjuvant Temozolomide. J. Pers. Med. 2021, 11, 1145. [Google Scholar] [CrossRef]
- Stiefel, I.; Schröder, C.; Tanadini-Lang, S.; Pytko, I.; Vu, E.; Klement, R.J.; Guckenberger, M.; Andratschke, N. High-dose re-irradiation of intracranial lesions—Efficacy and safety including dosimetric analysis based on accumulated EQD2Gy dose EQD calculation. Clin. Transl. Radiat. Oncol. 2021, 27, 132–138. [Google Scholar] [CrossRef]
- Hau, P.; Baumgart, U.; Pfeifer, K.; Bock, A.; Jauch, T.; Dietrich, J.; Fabel, K.; Grauer, O.; Wismeth, C.; Klinkhammer-Schalke, M.; et al. Salvage therapy in patients with glioblastoma: Is there any benefit? Cancer 2003, 98, 2678–2686. [Google Scholar] [CrossRef]
- Kondziolka, D.; Flickinger, J.C.; Bissonette, D.J.; Bozik, M.; Lunsford, L.D. Survival benefit of stereotactic radiosurgery for patients with malignant glial neoplasms. Neurosurgery 1997, 41, 776–783, discussion 783–785. [Google Scholar] [CrossRef] [Green Version]
- Gutin, P.H.; Iwamoto, F.M.; Beal, K.; Mohile, N.A.; Karimi, S.; Hou, B.L.; Lymberis, S.; Yamada, Y.; Chang, J.; Abrey, L.E. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 156–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vordermark, D.; Kolbl, O.; Ruprecht, K.; Vince, G.H.; Bratengeier, K.; Flentje, M. Hypofractionated stereotactic re-irradiation: Treatment option in recurrent malignant glioma. BMC Cancer 2005, 5, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaprak, G.; Isık, N.; Gemici, C.; Pekyurek, M.; Bıcakcı, B.C.; Demircioglu, F.; Tatarlı, N. Stereotactic Radiotherapy in Recurrent Glioblastoma: A Valid Salvage Treatment Option. Ster. Funct. Neurosurg. 2020, 98, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.E.; Parker, E.C.; Rush, S.C.; Kalhorn, S.P.; Moshel, Y.A.; Narayana, A.; Donahue, B.; Golfinos, J.G. Efficacy of gamma knife radiosurgery for small-volume recurrent malignant gliomas after initial radical resection. World Neurosurg. 2011, 76, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Grosu, A.L.; Weber, W.A.; Franz, M.; Stärk, S.; Piert, M.; Thamm, R.; Gumprecht, H.; Schwaiger, M.; Molls, M.; Nieder, C. Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Klobukowski, L.; Falkov, A.; Chelimo, C.; Fogh, S.E. A Retrospective Review of Re-irradiating Patients’ Recurrent High-grade Gliomas. Clin. Oncol. R. Coll. Radiol. 2018, 30, 563–570. [Google Scholar] [CrossRef]
- Hasan, S.; Chen, E.; Lanciano, R.M.; Yang, J.; Hanlon, A.; Lamond, J.; Arrigo, S.; Ding, W.; Mikhail, M.; Ghaneie, A.; et al. Salvage Fractionated Stereotactic Radiotherapy with or without Chemotherapy and Immunotherapy for Recurrent Glioblastoma Multiforme: A Single Institution Experience. Front. Oncol. 2015, 5, 106. [Google Scholar] [CrossRef] [Green Version]
- Gorlia, T.; Stupp, R.; Brandes, A.A.; Rampling, R.R.; Fumoleau, P.; Dittrich, C.; Campone, M.M.; Twelves, C.C.; Raymond, E.; Hegi, M.E.; et al. New prognostic factors and calculators for outcome prediction in patients with recurrent glioblastoma: A pooled analysis of EORTC Brain Tumour Group phase I and II clinical trials. Eur. J. Cancer 2012, 48, 1176–1184. [Google Scholar] [CrossRef]
- Chapman, C.H.; Hara, J.H.; Molinaro, A.M.; Clarke, J.L.; Oberheim Bush, N.A.; Taylor, J.W.; Butowski, N.A.; Chang, S.M.; Fogh, S.E.; Sneed, P.K.; et al. Reirradiation of recurrent high-grade glioma and development of prognostic scores for progression and survival. Neurooncol. Pract. 2019, 6, 364–374. [Google Scholar] [CrossRef]
- Conti, A.; Pontoriero, A.; Arpa, D.; Siragusa, C.; Tomasello, C.; Romanelli, P.; Cardali, S.; Granata, F.; De Renzis, C.; Tomasello, F. Efficacy and toxicity of CyberKnife re-irradiation and “dose dense” temozolomide for recurrent gliomas. Acta Neurochir. 2012, 154, 203–209. [Google Scholar] [CrossRef]
- Flieger, M.; Ganswindt, U.; Schwarz, S.B.; Kreth, F.W.; Tonn, J.C.; la Fougère, C.; Ertl, L.; Linn, J.; Herrlinger, U.; Belka, C.; et al. Re-irradiation and bevacizumab in recurrent highgrade glioma: An effective treatment option. J. Neurooncol. 2014, 117, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, F.; Soon, Y.Y.; Leong, Y.H.; Koh, W.Y.; Vellayappan, B. Re-irradiation for recurrent glioblastoma (GBM): A systematic review and meta-analysis. J. Neuro-Oncol. 2019, 142, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Navarria, P.; Pessina, F.; Clerici, E.; Bellu, L.; Franzese, C.; Franzini, A.; Simonelli, M.; Bello, L.; Santoro, A.; Politi, L.S.; et al. Re-irradiation for recurrent high grade glioma (HGG) patients: Results of a single arm prospective phase 2 study. Radiother. Oncol. 2022, 167, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Barker, F.G.; Chang, S.M.; Gutin, P.H.; Malec, M.K.; McDermott, M.W.; Prados, M.D.; Wilson, C.B. Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery 1998, 42, 709–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harsh, G.R., IV; Levin, V.A.; Gutin, P.H.; Seager, M.; Silver, P.; Wilson, C.B. Reoperation for recurrent glioblastoma and anaplastic astrocytoma. Neurosurgery 1987, 21, 615–621. [Google Scholar] [CrossRef]
- Franceschi, E.; Bartolotti, M.; Tosoni, A.; Bartolini, S.; Sturiale, C.; Fioravanti, A.; Pozzati, E.; Galzio, R.; Talacchi, A.; Volpin, L.; et al. The effect of re-operation on survival in patients with recurrent glioblastoma. Anticancer Res. 2015, 35, 1743–1748. [Google Scholar]
- Park, J.K.; Hodges, T.; Arko, L.; Shen, M.; Dello Iacono, D.; McNabb, A.; Bailey, N.O.; Kreisl, T.N.; Iwamoto, F.M.; Sul, J.; et al. Scale to predict survival after surgery for recurrent glioblastoma multiforme. J. Clin. Oncol. 2010, 28, 3838–3843. [Google Scholar] [CrossRef]
- De Bonis, P.; Fiorentino, A.; Anile, C.; Balducci, M.; Pompucci, A.; Chiesa, S.; Sica, G.; Lama, G.; Maira, G.; Mangiola, A. The impact of repeated surgery and adjuvant therapy on survival for patients with recurrent glioblastoma. Clin. Neurol. Neurosurg. 2013, 115, 883–886. [Google Scholar] [CrossRef]
- Chun, S.J.; Park, S.H.; Park, C.K.; Kim, J.W.; Kim, T.M.; Choi, S.H.; Lee, S.T.; Kim, I.H. Survival gain with re-Op/RT for recurred high-grade gliomas depends upon risk groups. Radiother. Oncol. 2018, 128, 254–259. [Google Scholar] [CrossRef]
- Leone, A.; Colamaria, A.; Fochi, N.P.; Sacco, M.; Landriscina, M.; Parbonetti, G.; de Notaris, M.; Coppola, G.; De Santis, E.; Giordano, G.; et al. Recurrent Glioblastoma Treatment: State of the Art and Future Perspectives in the Precision Medicine Era. Biomedicines 2022, 10, 1927. [Google Scholar] [CrossRef]


| Number of patients | 30 | ||
| Gender | |||
| Male | 19 | (63%) | |
| Female | 11 | (37%) | |
| Age | |||
| Median (range) (years) | 54 | (36–76) | |
| <60 (years) | 22 | (73%) | |
| 60–65 (years) | 5 | (17%) | |
| >65 (years) | 3 | (10%) | |
| KPS score | |||
| Median (range) (%) | 80 | (50–90) | |
| <60% | 1 | (3%) | |
| 60–70% | 11 | (37%) | |
| >70% | 18 | (60%) | |
| RPA class | |||
| Median (range) | IV | (III–V) | |
| III | 6 | (20%) | |
| IV | 15 | (50%) | |
| V | 9 | (30%) | |
| Mass effect | |||
| Yes | 16 | (53%) | |
| No | 14 | (47%) | |
| Multifocal tumor | |||
| Yes | 3 | (10%) | |
| No | 27 | (90%) | |
| Tumor histology | |||
| Glioblastoma | 24 | (80%) | |
| Anaplastic Astrocytoma | 5 | (17%) | |
| Oligodendroglioma | 1 | (3%) | |
| MGMT methylation | |||
| Methylated | 10 | (33%) | |
| Unmethylated | 12 | (40%) | |
| Not Available | 8 | (27%) | |
| IDH Mutation | |||
| Mutated | 0 | (0%) | |
| Wild Type | 24 | (80%) | |
| Not Available | 6 | (20%) |
| Number of patients | 30 | |
| Surgery at diagnosis | ||
| Complete | 14 | (47%) |
| Incomplete | 13 | (43%) |
| Unresectable (biopsy) | 3 | (10%) |
| Median time between surgery and adjuvant therapy (range) | 8 weeks | 2–18 weeks |
| Primary/Adjuvant RT + TMZ | ||
| Hypofractionated | 9 | (30%) |
| Conventional fractionated | 21 | (70%) |
| Median time between adjuvant therapy and recurrence (range) | 8 months | 2–27 months |
| Surgery at recurrence | ||
| Performed | 6 | (20%) |
| Not performed | 24 | (80%) |
| reRT at recurrence | ||
| Performed | 30 | (100%) |
| Systemic therapy at recurrence | ||
| Regorafenib | 17 | (57%) |
| Fotemustine | 5 | (16%) |
| Bevacizumab | 2 | (7%) |
| Metronomic Temozolomide | 2 | (7%) |
| None | 4 | (13%) |
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Sex | 5.88 | 0.75–46.06 | 0.09 | - | - | - |
| Age (≥54 years) | 3.8 | 0.9–16.02 | 0.02 | 7.98 | 0.76–83.0 | 0.05 |
| KPS (≤80%) | 0.65 | 0.15–2.76 | 0.5 | - | - | - |
| RPA (≥IV) | 6.64 | 0.83–52.69 | 0.05 | 3.78 | 0.42–33.64 | 0.2 |
| Adjuvant RT dose (hypofractionated) | 3.66 | 0.7–19.01 | 0.12 | - | - | - |
| Resection (incomplete) | 5.32 | 1.1–27.48 | 0.04 | 14.65 | 1.13–190.0 | 0.04 |
| Diagnosis (GB) | 0.67 | 0.14–3.16 | 0.61 | - | - | - |
| MGMT methylation (absent) | 0.56 | 0.11–2.81 | 0.48 | - | - | - |
| BED of RT dose at recurrence (>40 Gy) | 0.42 | 0.08–1.99 | 0.27 | - | - | - |
| PTV (>14.5 cc) | 1.09 | 0.33–3.62 | 0.88 | - | - | - |
| More local treatment | 0.37 | 0.09–1.44 | 0.15 | - | - | - |
| Recurrence time (≥8 months) | 1.69 | 0.5–5.7 | 0.39 | - | - | - |
| Progression time (>11 months) | 0.12 | 0.02–0.64 | 0.01 | 0.15 | 0.02–0.82 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregucci, F.; Surgo, A.; Carbonara, R.; Laera, L.; Ciliberti, M.P.; Gentile, M.A.; Caliandro, M.; Sasso, N.; Bonaparte, I.; Fanelli, V.; et al. Radiosurgery and Stereotactic Brain Radiotherapy with Systemic Therapy in Recurrent High-Grade Gliomas: Is It Feasible? Therapeutic Strategies in Recurrent High-Grade Gliomas. J. Pers. Med. 2022, 12, 1336. https://doi.org/10.3390/jpm12081336
Gregucci F, Surgo A, Carbonara R, Laera L, Ciliberti MP, Gentile MA, Caliandro M, Sasso N, Bonaparte I, Fanelli V, et al. Radiosurgery and Stereotactic Brain Radiotherapy with Systemic Therapy in Recurrent High-Grade Gliomas: Is It Feasible? Therapeutic Strategies in Recurrent High-Grade Gliomas. Journal of Personalized Medicine. 2022; 12(8):1336. https://doi.org/10.3390/jpm12081336
Chicago/Turabian StyleGregucci, Fabiana, Alessia Surgo, Roberta Carbonara, Letizia Laera, Maria Paola Ciliberti, Maria Annunziata Gentile, Morena Caliandro, Nicola Sasso, Ilaria Bonaparte, Vincenzo Fanelli, and et al. 2022. "Radiosurgery and Stereotactic Brain Radiotherapy with Systemic Therapy in Recurrent High-Grade Gliomas: Is It Feasible? Therapeutic Strategies in Recurrent High-Grade Gliomas" Journal of Personalized Medicine 12, no. 8: 1336. https://doi.org/10.3390/jpm12081336