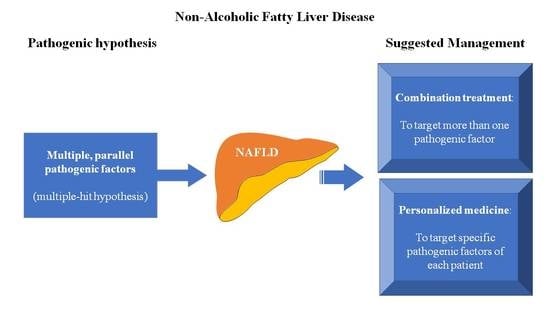
Combination Therapies for Nonalcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. Major Contributors to the Pathophysiology of NAFLD
2.1. Genetic Factors
2.2. Intrahepatic Lipid Accumulation
2.3. Mitochondrial Dysfunction and Endoplasmic Reticulum Stress
2.4. Adipose Tissue Dysfunction
2.5. Gut Microbiota Dysbiosis
2.6. Other Pathogenic Contributors
3. Combination Treatment of NAFLD
3.1. Anti-Obesity Medications
3.2. Ursodeoxycholic Acid
3.3. Farnesoid X Receptor Agonists
3.4. Fatty Acid Synthesis Enzyme Inhibitors
3.5. Anti-Apoptotic Medications
3.6. Anti-Oxidant Medications
3.7. Hypolipidemic Medications
3.8. Mineralocorticoid Receptor Antagonists
3.9. Anti-Diabetic Medications
4. Closing Remarks
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Polyzos, S.A.; Mantzoros, C.S. Nonalcoholic fatty future disease. Metabolism 2016, 65, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Wattacheril, J. Extrahepatic Manifestations of Nonalcoholic Fatty Liver Disease. Gastroenterol. Clin. N. Am. 2020, 49, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Scorletti, E.; Mosca, A.; Alisi, A.; Byrne, C.D.; Targher, G. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism 2020, 111, 154170. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kechagias, S.; Tsochatzis, E.A. Review article: Non-alcoholic fatty liver disease and cardiovascular diseases: Associations and treatment considerations. Aliment. Pharmacol. Ther. 2021, 54, 1013–1025. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; Sanyal, A.; Neuschwander-Tetri, B.; Tiribelli, C.; Kleiner, D.E.; Brunt, E.; Bugianesi, E.; Yki-Järvinen, H.; et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Mantzoros, C.S. Making progress in nonalcoholic fatty liver disease (NAFLD) as we are transitioning from the era of NAFLD to dys-metabolism associated fatty liver disease (DAFLD). Metabolism 2020, 111S, 154318. [Google Scholar] [CrossRef]
- Fazel, Y.; Koenig, A.B.; Sayiner, M.; Goodman, Z.D.; Younossi, Z.M. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism 2016, 65, 1017–1025. [Google Scholar] [CrossRef] [Green Version]
- Schattenberg, J.M.; Lazarus, J.V.; Newsome, P.N.; Serfaty, L.; Aghemo, A.; Augustin, S.; Tsochatzis, E.; de Ledinghen, V.; Bugianesi, E.; Romero-Gomez, M.; et al. Disease burden and economic impact of diagnosed non-alcoholic steatohepatitis in five European countries in 2018: A cost-of-illness analysis. Liver Int. 2021, 41, 1227–1242. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kang, E.S.; Boutari, C.; Rhee, E.J.; Mantzoros, C.S. Current and emerging pharmacological options for the treatment of nonalcoholic steatohepatitis. Metabolism 2020, 111, 154203. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Zavos, C.; Deretzi, G. Nonalcoholic fatty liver disease: Multimodal treatment options for a pathogenetically multiple-hit disease. J. Clin. Gastroenterol. 2012, 46, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Anastasiadis, S.; Doulberis, M.; Katsinelos, P. Nonalcoholic fatty liver disease: Is it time for combination treatment and a diabetes-like approach? Hepatology 2018, 68, 389. [Google Scholar] [CrossRef] [Green Version]
- Bedossa, P. Pathology of non-alcoholic fatty liver disease. Liver Int. 2017, 37 (Suppl. 1), 85–89. [Google Scholar] [CrossRef] [Green Version]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Zavos, C. Nonalcoholic fatty liver disease: The pathogenetic roles of insulin resistance and adipocytokines. Curr. Mol. Med. 2009, 9, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Adipose tissue, obesity and non-alcoholic fatty liver disease. Minerva Endocrinol. 2016, 42, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Taylor, R.J.; Bayliss, S.; Hagström, H.; Nasr, P.; Schattenberg, J.M.; Ishigami, M.; Toyoda, H.; Wong, V.W.-S.; Peleg, N.; et al. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1611–1625.e12. [Google Scholar] [CrossRef] [Green Version]
- Polyzos, S.A.; Mantzoros, C.S. An update on the validity of irisin assays and the link between irisin and hepatic metabolism. Metabolism 2015, 64, 937–942. [Google Scholar] [CrossRef]
- Grimaudo, S.; Pipitone, R.M.; Pennisi, G.; Celsa, C.; Cammà, C.; Di Marco, V.; Barcellona, M.R.; Boemi, R.; Enea, M.; Giannetti, A.; et al. Association Between PNPLA3 rs738409 C>G Variant and Liver-Related Outcomes in Patients With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2020, 18, 935–944.e3. [Google Scholar] [CrossRef]
- Eslam, M.; Valenti, L.; Romeo, S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J. Hepatol. 2018, 68, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; George, J. Genetic and epigenetic mechanisms of NASH. Hepatol. Int. 2016, 10, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Dixon, L.J.; Feldstein, A.E. Lipotoxicity in nonalcoholic fatty liver disease: Not all lipids are created equal. Expert Rev. Gastroenterol. Hepatol. 2009, 3, 445–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Human fatty liver disease: Old questions and new insights. Science 2011, 332, 1519–1523. [Google Scholar] [CrossRef] [Green Version]
- Lewis, G.F.; Carpentier, A.; Adeli, K.; Giacca, A. Disordered Fat Storage and Mobilization in the Pathogenesis of Insulin Resistance and Type 2 Diabetes. Endocr. Rev. 2002, 23, 201–229. [Google Scholar] [CrossRef]
- Makri, E.; Goulas, A.; Polyzos, S.A. Epidemiology, Pathogenesis, Diagnosis and Emerging Treatment of Nonalcoholic Fatty Liver Disease. Arch. Med. Res. 2021, 52, 25–37. [Google Scholar] [CrossRef]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.-H.; et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef] [Green Version]
- Nagai, Y.; Yonemitsu, S.; Erion, D.M.; Iwasaki, T.; Stark, R.; Weismann, D.; Dong, J.; Zhang, D.; Jurczak, M.J.; Löffler, M.G.; et al. The role of peroxisome proliferator-activated receptor γ coactivator-1 β in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 2009, 9, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Cobbina, E.; Akhlaghi, F. Non-alcoholic fatty liver disease (NAFLD)—Pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab. Rev. 2017, 49, 197–211. [Google Scholar] [CrossRef]
- Lebeaupin, C.; Vallée, D.; Hazari, Y.; Hetz, C.; Chevet, E.; Bailly-Maitre, B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 927–947. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.; Banini, B.A.; Cazanave, S.C.; Sanyal, A.J. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism 2016, 65, 1049–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vachliotis, I.; Goulas, A.; Papaioannidou, P.; Polyzos, S.A. Nonalcoholic fatty liver disease: Lifestyle and quality of life. Hormones 2022, 21, 41–49. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Adipokines in nonalcoholic fatty liver disease. Metabolism 2016, 65, 1062–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilg, H.; Hotamisligil, G.S. Nonalcoholic fatty liver disease: Cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology 2006, 131, 934–945. [Google Scholar] [CrossRef] [Green Version]
- Polyzos, S.A.; Kountouras, J.; Zavos, C.; Tsiaousi, E. The role of adiponectin in the pathogenesis and treatment of non-alcoholic fatty liver disease. Diabetes Obes. Metab. 2010, 12, 365–383. [Google Scholar] [CrossRef]
- Bashiardes, S.; Shapiro, H.; Rozin, S.; Shibolet, O.; Elinav, E. Non-alcoholic fatty liver and the gut microbiota. Mol. Metab. 2016, 5, 782–794. [Google Scholar] [CrossRef]
- Benedict, M.; Zhang, X. Non-alcoholic fatty liver disease: An expanded review. World J. Hepatol. 2017, 9, 715–732. [Google Scholar] [CrossRef]
- Polyzos, S.A. Endocrine and metabolic disorders interplaying with non-alcoholic fatty liver disease. Minerva Endocrinol. 2017, 42, 89–91. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Papatheodorou, A.; Patsiaoura, K.; Katsiki, E.; Zafeiriadou, E.; Zavos, C.; Anastasiadou, K.; Terpos, E. Helicobacter pylori infection in patients with nonalcoholic fatty liver disease. Metabolism 2013, 62, 121–126. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Deretzi, G.; Zavos, C.; Mantzoros, C.S. The emerging role of endocrine disruptors in pathogenesis of insulin resistance: A concept implicating nonalcoholic fatty liver disease. Curr. Mol. Med. 2012, 12, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Treviño, L.S.; Katz, T.A. Endocrine Disruptors and Developmental Origins of Nonalcoholic Fatty Liver Disease. Endocrinology 2018, 159, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Fecht, W.; Brunt, E.M.; Neuschwander-Tetri, B.A. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology 2009, 49, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Dufour, J.-F.; Oneta, C.M.; Gonvers, J.-J.; Bihl, F.; Cerny, A.; Cereda, J.-M.; Zala, J.-F.; Helbling, B.; Steuerwald, M.; Zimmermann, A.; et al. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 2006, 4, 1537–1543. [Google Scholar] [CrossRef]
- Pietu, F.; Guillaud, O.; Walter, T.; Vallin, M.; Hervieu, V.; Scoazec, J.Y.; Dumortier, J. Ursodeoxycholic acid with vitamin E in patients with nonalcoholic steatohepatitis: Long-term results. Clin. Res. Hepatol. Gastroenterol. 2012, 36, 146–155. [Google Scholar] [CrossRef]
- Madan, K.; Batra, Y.; Gupta, D.S.; Chander, B.; Anand Rajan, K.D.; Singh, R.; Panda, S.K.; Acharya, S.K. Vitamin E-based therapy is effective in ameliorating transaminasemia in nonalcoholic fatty liver disease. Indian J. Gastroenterol. 2005, 24, 251–255. [Google Scholar]
- Loomba, R.; Noureddin, M.; Kowdley, K.V.; Kohli, A.; Sheikh, A.; Neff, G.; Bhandari, B.R.; Gunn, N.; Caldwell, S.H.; Goodman, Z.; et al. Combination Therapies Including Cilofexor and Firsocostat for Bridging Fibrosis and Cirrhosis Attributable to NASH. Hepatology 2021, 73, 625–643. [Google Scholar] [CrossRef]
- Loomba, R.; Lawitz, E.; Mantry, P.S.; Jayakumar, S.; Caldwell, S.H.; Arnold, H.; Diehl, A.M.; Djedjos, C.S.; Han, L.; Myers, R.P.; et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology 2018, 67, 549–559. [Google Scholar] [CrossRef]
- Harrison, S.A.; Torgerson, S.; Hayashi, P.; Ward, J.; Schenker, S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am. J. Gastroenterol. 2003, 98, 2485–2490. [Google Scholar] [CrossRef]
- Nobili, V.; Manco, M.; Devito, R.; Di Ciommo, V.; Comparcola, D.; Sartorelli, M.R.; Piemonte, F.; Marcellini, M.; Angulo, P. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: A randomized, controlled trial. Hepatology 2008, 48, 119–128. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Masarone, M.; Gravina, A.G.; Di Sarno, R.; Tuccillo, C.; Cossiga, V.; Lama, S.; Stiuso, P.; Morisco, F.; et al. Evaluation of the Effect Derived from Silybin with Vitamin D and Vitamin E Administration on Clinical, Metabolic, Endothelial Dysfunction, Oxidative Stress Parameters, and Serological Worsening Markers in Nonalcoholic Fatty Liver Disease Patients. Oxid. Med. Cell. Longev. 2019, 2019, 8742075. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, C.; Andreone, P.; Brisc, C.; Brisc, M.C.; Bugianesi, E.; Chiaramonte, M.; Cursaro, C.; Danila, M.; de Sio, I.; Floreani, A.; et al. Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: A randomized controlled trial. Free Radic. Biol. Med. 2012, 52, 1658–1665. [Google Scholar] [CrossRef] [Green Version]
- Athyros, V.G.; Mikhailidis, D.P.; Didangelos, T.P.; Giouleme, O.I.; Liberopoulos, E.N.; Karagiannis, A.; Kakafika, A.I.; Tziomalos, K.; Burroughs, A.K.; Elisaf, M.S. Effect of multifactorial treatment on non-alcoholic fatty liver disease in metabolic syndrome: A randomised study. Curr. Med. Res. Opin. 2006, 22, 873–883. [Google Scholar] [CrossRef]
- Foster, T.; Budoff, M.J.; Saab, S.; Ahmadi, N.; Gordon, C.; Guerci, A.D. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: The St Francis Heart Study randomized clinical trial. Am. J. Gastroenterol. 2011, 106, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, C.; Carpino, G.; De Vito, R.; De Stefanis, C.; Alisi, A.; Cianfarani, S.; Overi, D.; Mosca, A.; Stronati, L.; Cucchiara, S.; et al. Docosahexanoic Acid Plus Vitamin D Treatment Improves Features of NAFLD in Children with Serum Vitamin D Deficiency: Results from a Single Centre Trial. PLoS ONE 2016, 11, e0168216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zöhrer, E.; Alisi, A.; Jahnel, J.; Mosca, A.; Della Corte, C.; Crudele, A.; Fauler, G.; Nobili, V. Efficacy of docosahexaenoic acid–choline–vitamin E in paediatric NASH: A randomized controlled clinical trial. Appl. Physiol. Nutr. Metab. 2017, 42, 948–954. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S.; Polymerou, V.; Katsinelos, P. Effects of combined low-dose spironolactone plus vitamin E vs vitamin E monotherapy on insulin resistance, non-invasive indices of steatosis and fibrosis, and adipokine levels in non-alcoholic fatty liver disease: A randomized controlled trial. Diabetes Obes. Metab. 2017, 19, 1805–1809. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Mofrad, P.S.; Contos, M.J.; Sargeant, C.; Luketic, V.A.; Sterling, R.K.; Stravitz, R.T.; Shiffman, M.L.; Clore, J.; Mills, A.S. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 2004, 2, 1107–1115. [Google Scholar] [CrossRef]
- Riche, D.M.; Fleming, J.W.; Malinowski, S.S.; Black, C.A.; Miller, K.H.; Wofford, M.R. Resistant nonalcoholic fatty liver disease amelioration with rosuvastatin and pioglitazone combination therapy in a patient with metabolic syndrome. Ann. Pharmacother. 2014, 48, 137–141. [Google Scholar] [CrossRef]
- Shah, P.K.; Mudaliar, S.; Chang, A.R.; Aroda, V.; Andre, M.; Burke, P.; Henry, R.R. Effects of intensive insulin therapy alone and in combination with pioglitazone on body weight, composition, distribution and liver fat content in patients with type 2 diabetes. Diabetes Obes. Metab. 2011, 13, 505–510. [Google Scholar] [CrossRef] [Green Version]
- Zib, I.; Jacob, A.N.; Lingvay, I.; Salinas, K.; McGavock, J.M.; Raskin, P.; Szczepaniak, L.S. Effect of Pioglitazone Therapy on Myocardial and Hepatic Steatosis in Insulin-Treated Patients with Type 2 Diabetes. J. Investig. Med. 2007, 55, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.M.; Jones, F.J.; Shaw, J.C.; Williams, C.D.; Ward, J.A.; Harrison, S.A. Rosiglitazone versus rosiglitazone and metformin versus rosiglitazone and losartan in the treatment of nonalcoholic steatohepatitis in humans: A 12-month randomized, prospective, open- label trial. Hepatology 2011, 54, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Omer, Z.; Cetinkalp, S.; Akyildiz, M.; Yilmaz, F.; Batur, Y.; Yilmaz, C.; Akarca, U. Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2010, 22, 18–23. [Google Scholar] [CrossRef]
- Lingvay, I.; Roe, E.D.; Duong, J.; Leonard, D.; Szczepaniak, L.S. Effect of insulin versus triple oral therapy on the progression of hepatic steatosis in type 2 diabetes. J. Investig. Med. 2012, 60, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Katoh, S.; Hata, S.; Matsushima, M.; Ikemoto, S.; Inoue, Y.; Yokoyama, J.; Tajima, N. Troglitazone prevents the rise in visceral adiposity and improves fatty liver associated with sulfonylurea therapy—a randomized controlled trial. Metabolism 2001, 50, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Sturm, N.; Bronowicki, J.P.; Maynard-Muet, M.; Tran, A.; Heluwaert, F.; Plages, A.; Zarski, J.P. Metformin plus pentoxifylline versus prescriptive diet in non-alcoholic steatohepatitis (NASH): A randomized controlled pilot trial. Gastroenterol. Clin. Biol. 2009, 33, 984–986. [Google Scholar] [CrossRef] [PubMed]
- Sathyanarayana, P.; Jogi, M.; Muthupillai, R.; Krishnamurthy, R.; Samson, S.L.; Bajaj, M. Effects of combined exenatide and pioglitazone therapy on hepatic fat content in type 2 diabetes. Obesity 2011, 19, 2310–2315. [Google Scholar] [CrossRef]
- Shao, N.; Kuang, H.Y.; Hao, M.; Gao, X.Y.; Lin, W.J.; Zou, W. Benefits of exenatide on obesity and non-alcoholic fatty liver disease with elevated liver enzymes in patients with type 2 diabetes. Diabetes/Metab. Res. Rev. 2014, 30, 521–529. [Google Scholar] [CrossRef]
- Harreiter, J.; Just, I.; Leutner, M.; Bastian, M.; Brath, H.; Schelkshorn, C.; Klepochova, R.; Krššák, M.; Kautzky-Willer, A. Combined exenatide and dapagliflozin has no additive effects on reduction of hepatocellular lipids despite better glycaemic control in patients with type 2 diabetes mellitus treated with metformin: EXENDA, a 24-week, prospective, randomized, placebo-controlled pilot trial. Diabetes Obes. Metab. 2021, 23, 1129–1139. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Repetto, E.; Guja, C.; Hardy, E.; Han, J.; Jabbour, S.A.; Ferrannini, E. Exenatide and dapagliflozin combination improves markers of liver steatosis and fibrosis in patients with type 2 diabetes. Diabetes Obes. Metab. 2020, 22, 393–403. [Google Scholar] [CrossRef]
- Eriksson, J.W.; Lundkvist, P.; Jansson, P.A.; Johansson, L.; Kvarnström, M.; Moris, L.; Miliotis, T.; Forsberg, G.B.; Risérus, U.; Lind, L.; et al. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: A double-blind randomised placebo-controlled study. Diabetologia 2018, 61, 1923–1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, E.J.; Lee, D.H.; Jeon, H.J.; Oh, T.K. Long-term effectiveness and safety of quadruple combination therapy with empagliflozin versus dapagliflozin in patients with type 2 diabetes: 3-year prospective observational study. Diabetes Res. Clin. Pract. 2021, 182, 109123. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Jiang, T.; Kang, K.; Wen, Z. Efficacy of sitagliptin combined with metformin in the initial treatment of type 2 diabetes with non-alcoholic fatty liver. Chin. J. New Drugs 2014, 23, 215–218. [Google Scholar]
- Xiang, Z.; Chen, Y.P.; Ma, K.F.; Ye, Y.F.; Zheng, L.; Yang, Y.D.; Li, Y.M.; Jin, X. The role of ursodeoxycholic acid in non-alcoholic steatohepatitis: A systematic review. BMC Gastroenterol. 2013, 13, 140. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, P.; Cariou, B.; Lien, F.; Kuipers, F.; Staels, B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 2009, 89, 147–191. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M.; Ratziu, V.; Loomba, R.; Rinella, M.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; Geier, A.; Beckebaum, S.; Newsome, P.N.; et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: Interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019, 394, 2184–2196. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.; Harrison, S.A.; Elkhashab, M.; Trotter, J.F.; Herring, R.; Rojter, S.E.; Kayali, Z.; Wong, V.W.; Greenbloom, S.; Jayakumar, S.; et al. Cilofexor, a Nonsteroidal FXR Agonist, in Patients With Noncirrhotic NASH: A Phase 2 Randomized Controlled Trial. Hepatology 2020, 72, 58–71. [Google Scholar] [CrossRef]
- Stiede, K.; Miao, W.; Blanchette, H.S.; Beysen, C.; Harriman, G.; Harwood, H.J., Jr.; Kelley, H.; Kapeller, R.; Schmalbach, T.; Westlin, W.F. Acetyl-coenzyme A carboxylase inhibition reduces de novo lipogenesis in overweight male subjects: A randomized, double-blind, crossover study. Hepatology 2017, 66, 324–334. [Google Scholar] [CrossRef] [Green Version]
- Loomba, R.; Kayali, Z.; Noureddin, M.; Ruane, P.; Lawitz, E.J.; Bennett, M.; Wang, L.; Harting, E.; Tarrant, J.M.; McColgan, B.J.; et al. GS-0976 Reduces Hepatic Steatosis and Fibrosis Markers in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 1463–1473.e6. [Google Scholar] [CrossRef] [Green Version]
- Harrison, S.A.; Wong, V.W.; Okanoue, T.; Bzowej, N.; Vuppalanchi, R.; Younes, Z.; Kohli, A.; Sarin, S.; Caldwell, S.H.; Alkhouri, N.; et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. J. Hepatol. 2020, 73, 26–39. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, E.R., III; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Tziomalos, K.; Athyros, V.G.; Paschos, P.; Karagiannis, A. Nonalcoholic fatty liver disease and statins. Metabolism 2015, 64, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Mintziori, G.; Polyzos, S.A. Emerging and future therapies for nonalcoholic steatohepatitis in adults. Expert Opin. Pharmacother. 2016, 17, 1937–1946. [Google Scholar] [CrossRef]
- Papaefthymiou, A.; Doulberis, M.; Karafyllidou, K.; Chatzimichael, E.; Deretzi, G.; Exadaktylos, A.K.; Sampsonas, F.; Gelasakis, A.; Papamichos, S.I.; Kotronis, G.; et al. Effect of spironolactone on pharmacological treatment of nonalcoholic fatty liver disease. Minerva Endocrinol. 2021; online ahead of print. [Google Scholar] [CrossRef]
- Wada, T.; Kenmochi, H.; Miyashita, Y.; Sasaki, M.; Ojima, M.; Sasahara, M.; Koya, D.; Tsuneki, H.; Sasaoka, T. Spironolactone improves glucose and lipid metabolism by ameliorating hepatic steatosis and inflammation and suppressing enhanced gluconeogenesis induced by high-fat and high-fructose diet. Endocrinology 2010, 151, 2040–2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polyzos, S.A.; Kountouras, J.; Zafeiriadou, E.; Patsiaoura, K.; Katsiki, E.; Deretzi, G.; Zavos, C.; Tsarouchas, G.; Rakitzi, P.; Slavakis, A. Effect of spironolactone and vitamin E on serum metabolic parameters and insulin resistance in patients with nonalcoholic fatty liver disease. J. Renin. Angiotensin. Aldosterone Syst. 2011, 12, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Mantzoros, C.S. Adiponectin as a target for the treatment of nonalcoholic steatohepatitis with thiazolidinediones: A systematic review. Metabolism 2016, 65, 1297–1306. [Google Scholar] [CrossRef] [Green Version]
- Boettcher, E.; Csako, G.; Pucino, F.; Wesley, R.; Loomba, R. Meta-analysis: Pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2012, 35, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Ratziu, V.; Charlotte, F.; Bernhardt, C.; Giral, P.; Halbron, M.; Lenaour, G.; Hartmann-Heurtier, A.; Bruckert, E.; Poynard, T.; LIDO Study Group. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: Results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology 2010, 51, 445–453. [Google Scholar] [CrossRef]
- Athyros, V.G.; Alexandrides, T.K.; Bilianou, H.; Cholongitas, E.; Doumas, M.; Ganotakis, E.S.; Goudevenos, J.; Elisaf, M.S.; Germanidis, G.; Giouleme, O.; et al. The use of statins alone, or in combination with pioglitazone and other drugs, for the treatment of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and related cardiovascular risk. An Expert Panel Statement. Metabolism 2017, 71, 17–32. [Google Scholar] [CrossRef]
- Yokohama, S.; Yoneda, M.; Haneda, M.; Okamoto, S.; Okada, M.; Aso, K.; Hasegawa, T.; Tokusashi, Y.; Miyokawa, N.; Nakamura, K. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology 2004, 40, 1222–1225. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.K.; Sakhuja, P.; Malhotra, V.; Sharma, B.C.; Sarin, S.K. Beneficial effects of pentoxifylline on hepatic steatosis, fibrosis and necroinflammation in patients with non-alcoholic steatohepatitis. J. Gastroenterol. Hepatol. 2007, 22, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Makri, E.; Kita, M.; Goulas, A.; Papaioannidou, P.; Efstathiadou, Z.A.; Adamidou, F.; Polyzos, S.A. Comparative effectiveness of glucagon-like peptide-1 receptor agonists versus dipeptidyl peptidase-4 inhibitors on noninvasive indices of hepatic steatosis and fibrosis in patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. 2020, 14, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.J.; Angus, P.W.; Yeomans, N.D. Incretin-based therapies for the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016, 31, 23–31. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; Abouda, G.; Aldersley, M.A.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.S.; Harrison, S.A. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- Makri, E.S.; Goulas, A.; Polyzos, S.A. Sodium-glucose co-transporter 2 inhibitors in nonalcoholic fatty liver disease. Eur. J. Pharmacol. 2021, 907, 174272. [Google Scholar] [CrossRef]
- Palmer, S.C.; Tendal, B.; Mustafa, R.A.; Vandvik, P.O.; Li, S.; Hao, Q.; Tunnicliffe, D.; Ruospo, M.; Natale, P.; Saglimbene, V.; et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: Systematic review and network meta-analysis of randomised controlled trials. BMJ 2021, 372, m4573. [Google Scholar] [CrossRef]
- Ekstedt, M.; Hagström, H.; Nasr, P.; Fredrikson, M.; Stål, P.; Kechagias, S.; Hultcrantz, R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef] [Green Version]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obeticholic acid for the treatment of nonalcoholic steatohepatitis: Expectations and concerns. Metabolism 2020, 104, 154144. [Google Scholar] [CrossRef] [PubMed]
- Makri, E.; Cholongitas, E.; Tziomalos, K. Emerging role of obeticholic acid in the management of nonalcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 9039–9043. [Google Scholar] [CrossRef]
- Pockros, P.J.; Fuchs, M.; Freilich, B.; Schiff, E.; Kohli, A.; Lawitz, E.J.; Hellstern, P.A.; Owens-Grillo, J.; Van Biene, C.; Shringarpure, R.; et al. CONTROL: A randomized phase 2 study of obeticholic acid and atorvastatin on lipoproteins in nonalcoholic steatohepatitis patients. Liver Int. 2019, 39, 2082–2093. [Google Scholar] [CrossRef] [PubMed]
- Venetsanaki, V.; Karabouta, Z.; Polyzos, S.A. Farnesoid X nuclear receptor agonists for the treatment of nonalcoholic steatohepatitis. Eur. J. Pharmacol. 2019, 863, 172661. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Tacke, F.; Sanyal, A.J.; Anstee, Q.M. Report on the AASLD/EASL joint workshop on clinical trial endpoints in NAFLD. J. Hepatol. 2019, 71, 823–833. [Google Scholar] [CrossRef] [Green Version]

| First Author, Year [Reference] 1 | Groups (N) | Patients’ Characteristics | Study Type; Duration (Weeks) | Change in LFTs (Within Combination Group) | Change in Steatosis (Within Combination Group) | Change in Inflammation (Within Combination Group) | Change in Fibrosis (Within Combination Group) | Change in Additional Parameters | Between-Group Difference(s) |
|---|---|---|---|---|---|---|---|---|---|
| Harrison, 2009 [43] | (1) Vitamin E 800 IU (18) vs. (2) orlistat 360 mg + vitamin E 800 IU (23) | Overweight biopsy-proven NASH patients | RCT; 36 | Yes (ALT, AST) | Yes (hepatic biopsy), only in the subgroup with weight lost ≥5% | Yes, only in the subgroup with weight lost ≥9% | No | NAS improvement, only in the subgroup with weight lost ≥9% | No |
| Dufour, 2006 [44] | (1) Placebo + placebo (15) vs. (2) UDCA 12–15 mg/kg + placebo (18) vs. (3) UDCA 12–15 mg/kg + vitamin E 800 IU (15) | Biopsy-proven NASH patients | RCT; 96 | Yes (ALT, AST) | Yes (hepatic biopsy) | No | No | - | ALT decrease in group 3 vs. groups 1 and 2; AST decrease in group 3 vs. group 1 |
| Pietu, 2012 [45] | (1) UDCA 1680 mg + vitamin E 555 IU (101) | Biopsy-proven NASH patients | Retrospective uncontrolled study; 192 | Yes (ALT, AST, γ-GT) | Yes (hepatic biopsy) in 3/10 patients | Yes (hepatic biopsy) in 3/10 patients | Yes (hepatic biopsy) in 4/10 patients | NAS improvement in 7/10 patients | Νo control group |
| Madan, 2005 [46] | (1) Lifestyle counseling (18) vs. (2) lifestyle counseling + UDCA 600 mg (12) vs. (3) lifestyle counseling + UDCA 600 mg + vitamin E 400 mg (12) | Biopsy-proven NAFLD patients | Retrospective comparative study; 24 | Yes (ALT, AST) | NA | NA | NA | - | ALT decrease in group 3 vs. group 1; higher percentage of patients normalized transaminases in group 3 vs. group 1 and 2 |
| Loomba, 2021 [47] | (1) Placebo (39) vs. (2) selonsertib 18 mg (39) vs. (3) cilofexor 30 mg (40) vs. (4) firsocostat 20 mg (40) vs. (5) cilofexor 30 mg + selonsertib 18 mg (79) vs. (6) firsocostat 20 mg + selonsertib 18 mg (77) vs. (7) cilofexor 30 mg + firsocostat 20 mg (78) | Biopsy-proven NASH patients with F3 or F4 | RCT; 48 | NA | ΝA | NA | NA | - | ALT decrease in group 7 vs. group 1; steatosis, inflammation and NAS improved in group 7 vs. group 1 (hepatic biopsy) |
| Loomba, 2018 [48] | (1) Selonsertib 6 mg (20) vs. (2) selonsertib 18 mg (22) vs. (3) simtuzumab 125 mg (10) vs. (4) selonsertib 6 mg + simtuzumab 125 mg (10) vs. (5) selonsertib 18 mg + simtuzumab 125 mg (10) | Biopsy-proven NASH patients with F2 or F3 | Open-label RCT; 24 | NA | NA | NA | Yes (hepatic biopsy) in 4/10 patients (group 4) and in 2/9 patients (group 5) | - | NA |
| Harrison, 2003 [49] | (1) Placebo (22) vs. (2) vitamin E 1000 IU + vitamin C 1000 mg (23) | Biopsy-proven NASH patients | RCT; 24 | No | NA | No | Yes (hepatic biopsy) | - | No |
| Nobili, 2008 [50] | (1) Placebo (28) vs. (2) vitamin E 600 IU + vitamin C 500 mg (25) | Biopsy-proven NAFLD children | Open-label RCT; 96 | Yes (ALT, AST) | Yes (hepatic biopsy) | Yes | No | NAS improvement | No |
| Federico, 2019 [51] | (1) No treatment (30) vs. (2) silybin-phospholipid complex 606 mg + vitamin D 20 mg + vitamin E 30 mg (60) | Biopsy-proven NAFLD patients | RCT; 24 (on treatment) + 24 (wash-out; no treatment) | NA | NA | NA | NA | - | Higher percentage of patients with ALT and γ-GT decrease in group 2 (only in 6 months); higher percentage of patients with steatosis improvement in group 2 (TE) |
| Loguercio, 2012 [52] | (1) Placebo (69) vs. (2) silybin 188 mg + phosphatidylcholine 388 mg + vitamin E 179 mg (69) | Biopsy-proven NAFLD patients | RCT; 48 | Yes (ALT, AST, γ-GT) | Yes (hepatic biopsy) | Yes | Yes | NAS improvement | γ-GT decrease in group 2 |
| Athyros, 2006 [53] | (1) Atorvastatin 20 mg (63) vs. (2) fenofibrate 200 mg (62) vs. (3) atorvastatin 20 mg + fenofibrate 200 mg (61) | Non-diabetic NAFLD patients with MetS | Open-label, randomized; 54 | Yes (ALT, AST, γ-GT) | Yes (US) | NA | NA | - | Higher percentage of patients with NAFLD resolution in groups 1 and 3 vs. group 2 |
| Foster, 2011 [54] | (1) Placebo (36) vs. (2) atorvastatin 20 mg + vitamin E 1000 IU + vitamin C 1 g (44) | NAFLD patients | RCT; 192 | NA | Yes (L/S ratio; CT) | NA | NA | - | Higher percentage of patients with NAFLD resolution in group 2 |
| Della-Corte, 2016 [55] | (1) Placebo (23) vs. (2) DHA 500 mg + vitamin D 800 IU (18) | Biopsy-proven NAFLD children | RCT; 24 (on treatment) + 24 (wash-out; no treatment) | Yes (ALT) | Yes (hepatic biopsy) | Yes | No | NAS improvement | ALT decreased in group 2 |
| Zöhrer, 2017 [56] | (1) Placebo (20) vs. (2) DHA 250 mg + choline 201 mg + vitamin E 39 IU (20) | Biopsy-proven NASH children | RCT; 48 | Yes (ALT) | Yes (hepatic biopsy) | Yes | No | NAS improvement | NA |
| Polyzos, 2017 [57] | (1) Vitamin E 400 IU (17) vs. (2) vitamin E 400 IU + spironolactone 25 mg (14) | Biopsy-proven NAFLD patients | Open-label RCT; 52 | No | Yes (NAFLD liver fat score) | NA | No (APRI) | - | No |
| Sanyal, 2004 [58] | (1) Vitamin E 400 IU (10) vs. (2) vitamin E 400 IU + pioglitazone 30 mg (10) | Non-diabetic, biopsy-proven NASH patients | RCT; 24 | NA | Yes (hepatic biopsy) | Yes | Yes | - | Steatosis, ballooning and inflammation improved in group 2 |
| Riche, 2014 [59] | Rosuvastatin 20 mg + pioglitazone 15 mg | NAFLD patients with obesity and T2DM | Case report; 36 | Yes (ALT, AST) | Yes (US) | NA | NA | - | NA |
| Shah, 2011 [60] | (1) Insulin + placebo (13) vs. (2) insulin + pioglitazone 45 mg (12) | Patients with obesity and T2DM | RCT; 12–16 | NA | No (L/S ratio; CT) | NA | NA | - | No |
| Zib, 2007 [61] | (1) Insulin (16) vs. (2) insulin + pioglitazone 30 mg (16) | Patients with T2DM | Open-label RCT; 24 | No | Yes (MRS) | NA | NA | - | No |
| Torres, 2011 [62] | (1) Rosiglitazone 8 mg (31) vs. (2) rosiglitazone 8 mg + metformin 1000 mg (37) vs. (3) rosiglitazone 8 mg + losartan 50 mg (40) | Biopsy-proven NASH patients | Open-label RCT; 48 | Yes (ALT, AST) | Yes, in the subgroup of patients with NASH (hepatic biopsy) | Yes, in the subgroup of patients with NASH | Yes, in the subgroup of patients with NASH | NAS improvement in the subgroup of patients with NASH | No |
| Omer, 2010 [63] | (1) Metformin 1700 mg (22) vs. (2) rosiglitazone 4 mg (20) vs. (3) metformin 1700 mg + rosiglitazone 4 mg (22) | Patients with NAS ≥ 5 | Open-label RCT; 48 | Yes (ALT, AST, γ-GT) | NA | NA | No (hepatic biopsy) | NAS improvement | NA |
| Lingvay, 2012 [64] | (1) Metformin 2000 mg + insulin (10) vs. (2) metformin 2000 mg + glyburide 2.5 mg + pioglitazone 45 mg (6) | Patients with T2DM (after a 3-month lead-in period of insulin + metformin treatment) | RCT; 124 | NA | No (MRS) | NA | NA | - | No |
| Katoh, 2001 [65] | (1) Glibenclamide 3.7 ± 2.7 mg (38) vs. (2) glibenclamide 4.1 ± 2.5 mg + troglitazone 400 mg (40) | Patients with T2DM | RCT; 24 | NA | NA | NA | NA | - | ALT, γ-GT decrease in group 2; steatosis improvement in group 2 (CT) |
| Sturm, 2009 [66] | (1) Diet (9) vs. (2) diet + metformin 1500 mg + pentoxifylline 12 mg (10) | Non-diabetic NASH patients | RCT; 48 | No | No (hepatic biopsy) | NA | No | - | No |
| Sathyanarayana, 2011 [67] | (1) Pioglitazone 45 mg (10) vs. (2) pioglitazone 45 mg + exenatide 20 μg (11) | Patients with T2DM | Open-label RCT; 50 | Yes (ALT, AST) | Yes (MRS) | NA | NA | - | ALT decrease in group 2; steatosis improvement in group 2 |
| Shao, 2014 [68] | (1) Insulin aspart + insulin glargine (30) vs. (2) exenatide 10 μg (4 weeks) followed by 20 μg (8 weeks) + insulin glargine (30) | NAFLD patients with obesity and T2DM | RCT; 12 | Yes (ALT, AST, γ-GT) | Yes (US) | NA | NA | - | ALT, AST, γ-GT decrease in group 2; higher percentage of NAFLD regression in group 2 |
| Harreiter, 2021 [69] | (1) Placebo + dapagliflozin 10 mg (14) vs. (2) exenatide 2 mg + dapagliflozin 10 mg (16) | Patients with T2DM | RCT; 24 | Yes (ALT, AST) | Yes (MRS) | NA | NA | Yes (FLI) | No |
| Gastaldelli, 2020 [70] | (1) Exenatide 2 mg + placebo (227) vs. (2) dapagliflozin 10 mg + placebo (230) vs. (3) exenatide 2 mg + dapagliflozin 10 mg (228) | Patients with T2DM | Post hoc of RCT; 52 | Yes (ALT, γ-GT) | Yes (FLI and NAFLD liver fat score) | NA | Yes (NFS, FIB-4) | - | FLI and NAFLD liver fat score decrease in group 3 vs. group 1; ALT decrease in group 3 vs. group 1 |
| Eriksson, 2018 [71] | (1) Placebo (20) vs. (2) omega-3 4 gr (15) vs. (3) dapagliflozin 10 mg (20) vs. (4) dapagliflozin 10 mg + omega-3 4 gr (20) | NAFLD patients with T2DM | RCT; 12 | No | Yes (MRI-PDFF) | NA | NA | - | Steatosis improved in group 4 vs. group 1 |
| Ku, 2021 [72] | (1) Metformin 2 gr + glimepiride ≥ 6 mg + DPP-4i + empagliflozin 25 mg (185) vs. (2) metformin 2 gr + glimepiride ≥ 6 mg + DPP-4i + dapagliflozin 10 mg (177) | Patients with T2DM | Open-label prospective observational; 144 | NA | NA | NA | NA | - | No (LFTs) |
| Song, 2014 [73] | (1) Metformin 1500 mg + sitagliptin 100 mg vs. (2) metformin 1500 mg + glipizide 2.5–5 mg | NAFLD patients with T2DM | RCT; 16 | Yes (ALT, AST, γ-GT) | NA | NA | NA | - | ALT, AST, γ-GT decrease in group 1; steatosis improved in group 1 |
| Medications; Date of Enrollment Initiation (Date/Month/Year) 1 | Disease(s) | Estimated Enrollment (N) | Duration (Months) | Groups | Trial Identifier |
|---|---|---|---|---|---|
| Rosuvastatin and ezetimibe; 14 May 2018 | NAFLD/Dyslipidemia | 70 | 6 | Rosuvastatin vs. rosuvastatin + ezetimibe | NCT03434613 |
| Tropifexor and cenicriviroc; 11 September 2018 | NASH | 200 | 12 | Tropifexor vs. cenicriviroc vs. tropifexor + cenicriviroc | NCT03517540 |
| Pioglitazone and empagliflozin; 19 December 2018 | NAFLD/T2DM | 60 | 6 | Pioglitazone vs. empagliflozin vs. pioglitazone + empagliflozin | NCT03646292 |
| Garlic and silymarin and curcumin; 1 July 2019 | NAFLD | 60 | 3 | Garlic + silymarin + curcumin vs. placebo | IRCT20190602043787N1 |
| Tropifexor and licogliflozin; 11 December 2019 | NASH | 380 | 12 | Tropifexor + licogliflozin vs. tropifexor + placebo vs. licogliflozin + placebo vs. placebo + placebo | NCT04065841 |
| Saroglitazar and vitamin E; 16 December 2019 | NAFLD | 200 | 6 | Saroglitazar vs. vitamin E vs. saroglitazar + vitamin E vs. lifestyle modifications | CTRI/2019/12/022339 |
| Elobixibat and cholestyramine; 29 January 2020 | NAFLD/NASH | 100 | 4 | Elobixibat + cholestyramine vs. elobixibat + placebo vs. placebo + cholestyramine vs. placebo + placebo | NCT04235205 |
| LYS006 and tropifexor; 4 June 2020 | NAFLD/NASH | 250 | 5 | LYS006 vs. LYS006 + tropifexor | NCT04147195 |
| MET409 and empagliflozin; 15 December 2020 | NASH/T2DM | 120 | 3 | MET409 vs. placebo vs. MET409 + empagliflozin vs. placebo + empagliflozin | NCT04702490 |
| Empagliflozin and semaglutide; 26 March 2021 | NAFLD/NASH/T2DM | 192 | 12 | Empagliflozin + semaglutide vs. empagliflozin + placebo vs. placebo + placebo | NCT04639414 |
| Luseogliflozin and semaglutide; 29 July 2021 | NASH/T2DM | 60 | 12 | Luseogliflozin + semaglutide vs. semaglutide | jRCTs061210009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makri, E.S.; Makri, E.; Polyzos, S.A. Combination Therapies for Nonalcoholic Fatty Liver Disease. J. Pers. Med. 2022, 12, 1166. https://doi.org/10.3390/jpm12071166
Makri ES, Makri E, Polyzos SA. Combination Therapies for Nonalcoholic Fatty Liver Disease. Journal of Personalized Medicine. 2022; 12(7):1166. https://doi.org/10.3390/jpm12071166
Chicago/Turabian StyleMakri, Evangelia S., Eleftheria Makri, and Stergios A. Polyzos. 2022. "Combination Therapies for Nonalcoholic Fatty Liver Disease" Journal of Personalized Medicine 12, no. 7: 1166. https://doi.org/10.3390/jpm12071166