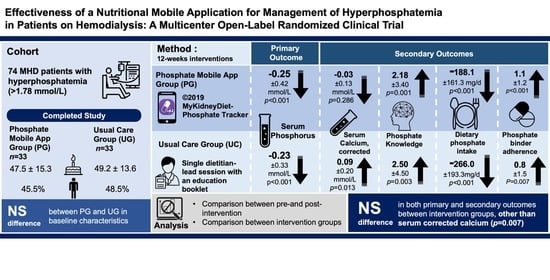
Effectiveness of a Nutritional Mobile Application for Management of Hyperphosphatemia in Patients on Hemodialysis: A Multicenter Open-Label Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subject Enrollment
2.2. Randomization
2.3. Study Groups
2.4. Outcome Measures
2.4.1. Biochemical Parameters
2.4.2. Patients’ Knowledge of Phosphorus Management
2.4.3. Dietary Intake
2.4.4. Phosphate Binder Adherence
2.4.5. Sociodemographic and Medical History
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Biochemical Parameters
3.3. Knowledge Score, Phosphate Binder Compliance, and Dietary Intakes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vervloet, M.G.; van Ballegooijen, A.J. Prevention and treatment of hyperphosphatemia in chronic kidney disease. Kidney Int. 2018, 93, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Garabed, E.; Norbert, L.; Bertram, L.K. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar]
- Isakova, T.; Nickolas, T.L.; Denburg, M.; Yarlagadda, S.; Weiner, D.E.; Gutiérrez, O.M.; Bansal, V.; Rosas, S.E.; Nigwekar, S.; Yee, J.; et al. KDOQI US commentary on the 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Am. J. Kidney Dis. 2017, 70, 737–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rroji, M.; Figurek, A.; Spasovski, G. Should we consider the cardiovascular system while evaluating CKD-MBD? Toxins 2020, 12, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St-Jules, D.E.; Woolf, K.; Pompeii, M.L.; Kalantar-Zadeh, K.; Sevick, M.A. Reexamining the Phosphorus–Protein Dilemma: Does Phosphorus Restriction Compromise Protein Status? J. Ren. Nutr. 2016, 26, 136–140. [Google Scholar] [CrossRef] [Green Version]
- Sahathevan, S.; Khor, B.H.; Ng, H.M.; Gafor, A.H.A.; Mat Daud, Z.A.; Mafra, D.; Karupaiah, T. Understanding development of malnutrition in hemodialysis patients: A narrative review. Nutrients 2020, 12, 3147. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Wang, A.Y.M.; Akizawa, T.; Bavanandan, S.; Hamano, T.; Liew, A.; Lu, K.C.; Lumlertgul, D.; Oh, K.H.; Zhao, M.H.; Fung, S.K.S.; et al. 2017 kidney disease: Improving global outcomes (KDIGO) chronic kidney disease–mineral and bone disorder (CKD-MBD) guideline update implementation: Asia summit conference report. Kidney Int. Rep. 2019, 4, 1523–1537. [Google Scholar] [CrossRef] [Green Version]
- Leung, S.; McCormick, B.; Wagner, J.; Biyani, M.; Lavoie, S.; Imtiaz, R.; Zimmerman, D. Meal phosphate variability does not support fixed dose phosphate binder schedules for patients treated with peritoneal dialysis: A prospective cohort study. BMC Nephrol. 2015, 16, 1–6. [Google Scholar] [CrossRef]
- Wang, A.Y.M.; Okpechi, I.G.; Ye, F.; Kovesdy, C.P.; Brunori, G.; Burrowes, J.D.; Campbell, K.; Damster, S.; Fouque, D.; Friedman, A.N.; et al. Assessing global kidney nutrition care. Clin. J. Am. Soc. Nephrol. 2022, 17, 38–52. [Google Scholar] [CrossRef]
- Osman, M.A.; Alrukhaimi, M.; Ashuntantang, G.E.; Bellorin-Font, E.; Gharbi, M.B.; Braam, B.; Courtney, M.; Feehally, J.; Harris, D.C.; Jha, V.; et al. Global nephrology workforce: Gaps and opportunities toward a sustainable kidney care system. Kidney Int. Suppl. 2018, 8, 52–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brauer, A.; Waheed, S.; Singh, T.; Maursetter, L. Improvement in hyperphosphatemia using phosphate education and planning talks. J. Ren. Nutr. 2019, 29, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Joson, C.G.; Henry, S.L.; Kim, S.; Cheung, M.Y.; Parab, P.; Abcar, A.C.; Jacobsen, S.J.; Morisky, D.E.; Sim, J.J. Patient-reported factors associated with poor phosphorus control in a maintenance hemodialysis population. J. Ren. Nutr. 2016, 26, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Khor, B.H.; Chinna, K.; Gafor, A.H.A.; Morad, Z.; Ahmad, G.; Bavanandam, S.; Visvanathan, R.; Yahya, R.; Goh, B.L.; Bee, B.C.; et al. The state of nutrition care in outpatient hemodialysis settings in Malaysia: A nationwide survey. BMC Health Serv. Res. 2018, 18, 939. [Google Scholar] [CrossRef]
- Sualeheen, A.; Khor, B.H.; Balasubramanian, G.V.; Sahathevan, S.; Ali, M.S.M.; Narayanan, S.S.; Chinna, K.; Daud, Z.A.M.; Khosla, P.; Gafor, A.H.A.; et al. Habitual dietary patterns of patients on hemodialysis indicate nutritional risk. J. Ren. Nutr. 2020, 30, 322–332. [Google Scholar] [CrossRef]
- Sahathevan, A.; Khor, B.H.; Sadu Singh, B.K.; Sabatino, A.; Fiaccadori, E.; Daud, Z.A.M.; Ali, M.S.; Narayanan, S.S.; Tallman, D.; Chinna, K.; et al. Association of ultrasound-derived metrics of the quadriceps muscle with protein energy wasting in hemodialysis patients: A multicenter cross-sectional study. Nutrients 2020, 12, 3597. [Google Scholar] [CrossRef]
- Anderson, C.A.; Nguyen, H.A. Nutrition education in the care of patients with chronic kidney disease and end-stage renal disease. Semin. Dial. 2018, 31, 115–121. [Google Scholar] [CrossRef]
- Teong, L.F.; Khor, B.H.; RadionPurba, K.; Gafor, A.H.A.; Goh, B.L.; Bee, B.C.; Yahya, R.; Bavanandan, S.; Ng, H.M.; Sahathevan, S.; et al. A Mobile App for Triangulating Strategies in Phosphate Education Targeting Patients with Chronic Kidney Disease in Malaysia: Development, Validation, and Patient Acceptance. Healthcare 2022, 10, 535. [Google Scholar] [CrossRef]
- Wong, H.S.; Goh, B.L. Twenty Forth Report of the Malaysian Dialysis and Transplant 2016; National Renal Registry-Clinical Research Centre/Ministry of Health: Kuala Lumpur, Malaysia, 2018. [Google Scholar]
- Yokum, D.; Glass, G.; Cheung, C.F.; Cunningham, J.; Fan, S.; Madden, A.M. Evaluation of a phosphate management protocol to achieve optimum serum phosphate levels in hemodialysis patients. J. Ren. Nutr. 2008, 18, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Tee, E.S.; Noor, M.I.; Azudin, M.N.; Idris, K. Nutrient Compositions of Malaysian Foods, 4th ed.; Institute for Medical Research: Kuala Lumpur, Malaysia, 1997. [Google Scholar]
- Health Promotion Board. Energy and Nutrient Composition of Foods Singapore. Health Promotion Board. Available online: https://focos.hpb.gov.sg/eservices/ENCF/ (accessed on 21 July 2021).
- Black, A.E. Critical evaluation of energy intake using the Goldberg cutoff for energy intake: Basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. 2000, 24, 1119–1130. [Google Scholar] [CrossRef] [Green Version]
- Morisky, D.E.; Green, L.W.; Levine, D.M. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986, 24, 67–74. [Google Scholar] [CrossRef] [PubMed]
- St-Jules, D.E.; Rozga, M.R.; Handu, D.; Carrero, J.J. Effect of phosphate-specific diet therapy on phosphate levels in adults undergoing maintenance hemodialysis: A systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 2021, 16, 107–120. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, C.F.; Crutzen, R.; Schols, J.M.; Halfens, R.J.; Karavetian, M. Adequate Management of Phosphorus in Patients Undergoing Hemodialysis Using a Dietary Smartphone App: Prospective Pilot Study. JMIR Form. Res. 2021, 5, e17858. [Google Scholar] [CrossRef] [PubMed]
- Farfan-Ruiz, A.C.; Czikk, D.; Leidecker, J.; Ramsay, T.; McCormick, B.; Wilson, K.; Zimmerman, D. Multidisciplinary Team versus a “Phosphate-Counting” App for Serum Phosphate Control: A Randomized Controlled Trial. Kidney360 2021, 2, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.C.; Chang, Y.P.; Lin, S.C.; Lin, C.; Hsu, P.H.; Hsu, Y.J.; Wu, T.J. Effects of Individualized Dietary Phosphate Control Program With a Smartphone Application in Hemodialysis Patients in Taiwan. Biol. Res. Nurs. 2021, 23, 375–381. [Google Scholar] [CrossRef]
- Elder, G.J. Calcium-based phosphate binders; down, but not out. Nephrol. Dial. Transplant. 2017, 32, 5–8. [Google Scholar] [CrossRef]
- Reid, I.R.; Gamble, G.D.; Bolland, M.J. Circulating calcium concentrations, vascular disease and mortality: A systematic review. J. Intern. Med. 2016, 279, 524–540. [Google Scholar] [CrossRef] [Green Version]
- Khor, B.H.; Sualeheen, A.; Sahathevan, S.; Chinna, K.; Gafor, A.H.A.; Bavanandan, S.; Goh, B.L.; Morad, Z.; Daud, Z.A.M.; Khosla, P.; et al. Association of dietary patterns with serum phosphorus in maintenance haemodialysis patients: A cross-sectional study. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Shinaberger, C.S.; Greenland, S.; Kopple, J.D.; Van Wyck, D.; Mehrotra, R.; Kovesdy, C.P.; Kalantar-Zadeh, K. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am. J. Clin. Nutr. 2008, 88, 1511–1518. [Google Scholar] [CrossRef] [Green Version]
- Friedman, A.N.; Fadem, S.Z. Reassessment of albumin as a nutritional marker in kidney disease. J. Am. Soc. Nephrol. 2010, 21, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Byrne, F.N.; Gillman, B.; Kiely, M.; Bowles, M.; Connolly, P.; Earlie, J.; Murphy, J.; Rennick, T.; O’Reilly, E.; Shiely, F.; et al. Translation of nutrient level recommendations to control serum phosphate into food-based advice. J. Ren. Nutr. 2021, 31, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Kopple, J.D.; Karupaiah, T.; Chan, M.; Burrowes, J.D.; Kirk, J.; Prest, M. Global renal internet course for dietitians (GRID course). J. Ren. Nutr. 2022, 32, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Pace, R.C.; Kirk, J. Academy of Nutrition and Dietetics and National Kidney Foundation: Revised 2020 standards of practice and standards of professional performance for registered dietitian nutritionists (competent, proficient, and expert) in nephrology nutrition. J. Ren. Nutr. 2021, 31, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Moore, L.W. Precision nutrition and personalized diet plan for kidney health and kidney disease management. J. Ren. Nutr. 2020, 30, 365–367. [Google Scholar] [CrossRef]
- Palmer, S.C.; Hayen, A.; Macaskill, P.; Pellegrini, F.; Craig, J.C.; Elder, G.J.; Strippoli, G.F. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: A systematic review and meta-analysis. JAMA 2011, 305, 1119–1127. [Google Scholar] [CrossRef]
- Young, E.W.; Akiba, T.; Albert, J.M.; McCarthy, J.T.; Kerr, P.G.; Mendelssohn, D.C.; Jadoul, M. Magnitude and impact of abnormal mineral metabolism in hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am. J. Kidney Dis. 2004, 44, 34–38. [Google Scholar] [CrossRef]



| Parameters | PG (n = 33) | UG (n = 33) | Between-Group p-Value | ||
|---|---|---|---|---|---|
| Mean ± SD | n (%) | Mean ± SD | n (%) | ||
| Age (year) | 47.5 ± 15.3 | 49.15 ± 13.63 | 0.636 a | ||
| Gender | 0.805 b | ||||
| Male | 15 (45.5) | 16 (48.5) | |||
| Female | 18 (54.5) | 17 (51.5) | |||
| Ethnicity | 0.356 b | ||||
| Malay | 18 (54.5) | 15 (45.5) | |||
| Chinese | 11 (33.3) | 13 (39.4) | |||
| Indian | 2 (6.1) | 5 (15.2) | |||
| Others | 2 (6.1) | 0 (0.0) | |||
| Marital status | 0.145 b | ||||
| Married | 6 (18.2) | 10 (30.3) | |||
| Single | 27 (81.8) | 21 (63.6) | |||
| Divorced | 0 (0.0) | 2 (6.1) | |||
| Education level | 0.087 b | ||||
| Diploma/Degree/Higher | 3 (9.1) | 8 (24.2) | |||
| Secondary | 12 (36.4) | 15 (45.5) | |||
| Primary | 18 (54.5) | 10 (30.3) | |||
| Monthly household income | 0.158 b | ||||
| Less than RM 500 | 15 (45.5) | 15 (45.5) | |||
| RM 501–1000 | 4 (12.1) | 6 (18.2) | |||
| RM 1001–2000 | 1 (3.0) | 5 (15.2) | |||
| RM 2001–3000 | 3 (9.1) | 4 (12.1) | |||
| RM 3001–4000 | 5 (15.2) | 0 (0.0) | |||
| RM 4001–5000 | 2 (6.1) | 2 (6.1) | |||
| More than RM5000 | 3 (9.1) | 1 (3.0) | |||
| Employment | 0.468 b | ||||
| Retired | 10 (30.3) | 11 (33.3) | |||
| Employed for wages | 5 (15.2) | 5 (15.2) | |||
| Self-employed | 3 (9.1) | 0 (0.0) | |||
| Housewife | 9 (27.3) | 8 (24.2) | |||
| Out of work | 4 (12.1) | 3 (9.1) | |||
| Student | 1 (3.0) | 1 (3.0) | |||
| Unable to work | 1 (3.0) | 5 (15.2) | |||
| Smartphone OS | 1.000 c | ||||
| Android | 31 (93.9) | 30 (90.9) | |||
| Apple iOS | 2 (6.1) | 3 (9.1) | |||
| Body mass index (kg/m2) | 22.4 ± 4.1 | 23.9 ± 4.1 | 0.152 a | ||
| ꝉ HD vintage (month) | 78 (119) | 49 (52) | 0.251 d | ||
| Kt/v (baseline) | 1.68 ± 0.30 | 1.69 ± 0.28 | 0.888 a | ||
| Kt/v (3-month) | 1.70 ± 0.28 | 1.71 ± 0.27 | 0.844 a | ||
| Phosphate binder | 0.311 b | ||||
| Calcium carbonate | 28 (84.8) | 29 (87.9) | |||
| Sevelamer carbonate | 1 (3.0) | 3 (9.1) | |||
| Lanthanum carbonate | 4 (12.1) | 1 (3.0) | |||
| Activated Vitamin D | 0.786 b | ||||
| Prescribed | 10 (30.3) | 9 (27.3) | |||
| Not prescribed | 23 (69.7) | 24 (72.7) | |||
| Calcimimetic | 0.492 b | ||||
| Prescribed | 2 (6.1) | 0 (0.0) | |||
| Not prescribed | 31 (93.9) | 33 (100.0) | |||
| Comorbidities | |||||
| Diabetes mellitus | 6 (18.2) | 13 (39) | 0.057 b | ||
| Hypertension | 22 (66.7) | 25 (75.8) | 0.415 b | ||
| Dyslipidemia | 12 (36.4) | 11 (33.3) | 0.796 b | ||
| Heart disease | 3 (9.1) | 2 (6.1) | 1.000 c | ||
| Anemia* | 9 (27.3) | 5 (15.2) | 0.228 b | ||
| Biochemical Parameters | |||||
| Phosphorus (mmol/L) | 2.34 ± 0.34 | 2.17 ± 0.34 | 0.053 a | ||
| Calcium, corrected (mmol/L) | 2.33 ± 0.23 | 2.23 ± 0.28 | 0.144 a | ||
| iPTH ꝉ (pmol/L) | 24.23 (18.81) | 26.25 (15.89) | 0.737 d | ||
| ALP ꝉ (U/L) | 150.16 (102.38) | 161.38 (139.55) | 0.720 a | ||
| Albumin (g/L) | 42.2 ± 3.3 | 41.1 ± 3.6 | 0.226 a | ||
| Total phosphate knowledge score | 9.6 ± 3.9 | 9.2± 4.1 | 0.690 | ||
| Overall phosphate management | 1.4 ± 1.0 | 1.1 ± 1.0 | 0.191 | ||
| Hyperphosphatemia consequences | 1.5 ± 1.0 | 1.6 ± 1.0 | 0.807 | ||
| Phosphate binder | 2.4 ± 1.2 | 2.2 ± 1.1 | 0.528 | ||
| Dietary phosphorus | 3.4 ± 1.8 | 3.8 ± 1.8 | 0.455 | ||
| Responsibility | 1.0 ± 0.2 | 0.7 ± 0.5 | 0.001 | ||
| Total MMAS-4 score | 2.1 ± 1.1 | 2.1 ± 1.1 | 1.000 | ||
| Dietary intakes | |||||
| Energy (kcal/day) | 1623 ± 318 | 1609 ± 412 | 0.889 | ||
| Energy (kcal/kg/day) | 29.1 ± 6.3 | 27.6 ± 7.7 | 0.459 | ||
| Protein (g/day) | 60.9 ± 15.2 | 60.2 ± 20.8 | 0.895 | ||
| Protein (g/kg/day) | 1.1 ± 0.3 | 1.0 ± 0.4 | 0.517 | ||
| Phosphorus (mg/day) | 817 ± 280 | 798 ± 267 | 0.804 | ||
| PPR (mg/g) | 13.5 ± 3.8 | 13.7 ± 3.7 | 0.878 | ||
| Parameters | PG (n = 33) | UG (n = 33) | Between-Group Change p-Value b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Change | Cohen’s d (95%CI) | Within-Group p-Value a | Before | After | Change | Cohen’s d (95%CI) | Within-Group p-Value a | ||
| Phosphorus (mmol/L) | 2.34 ± 0.34 | 2.08 ± 0.41 | −0.25 ± 0.42 | 0.61 (0.25–0.97) | 0.001 | 2.17 ± 0.34 | 1.95 ± 0.50 | −0.23 ± 0.33 | 0.69 (0.32–1.06) | <0.001 | 0.780 |
| Calcium, corrected (mmol/L) | 2.33 ± 0.23 | 2.30 ± 0.25 | −0.03 ± 0.13 | 0.19 (0.00–0.52) | 0.286 | 2.23 ± 0.28 | 2.33 ± 0.25 | 0.09 ± 0.20 | 0.46 (0.10–0.82) | 0.013 | 0.007 |
| iPTH ꝉ (pmol/L) | 24.23 (18.81) | 23.83 (19.02) | 0.40 ± 7.71 | 0.05 (0.00–0.34) | 0.815 d | 26.25 (15.89) | 23.39 (13.37) | 2.86 ± 6.29 | 0.45 (0.00–0.97) | 0.100 d | 0.317 c |
| Albumin (g/L) | 42.2 ± 3.3 | 41.1 ± 3.7 | −1.1 ± 2.7 | 0.42 (0.05–0.78) | 0.029 | 41.1 ± 3.6 | 40.5 ± 3.5 | −0.58 ± 3.52 | 0.16 (0.00–0.49) | 0.354 | 0.484 |
| Parameters | PG (n = 33) | UG (n = 33) | Between-Group Change p-Value b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Change | Cohen’s d (95%CI) | Within-Group p-Value a | Before | After | Change | Cohen’s d (95%CI) | Within-Group p-Value a | ||
| Phosphorus Knowledge Score | |||||||||||
| Total knowledge score | 9.6 ± 3.9 | 11.8 ± 2.5 | 2.2 ± 3.4 | 0.64 (0.26–1.01) | 0.001 | 9.2± 4.1 | 11.7 ± 3.3 | 2.5 ± 4.5 | 0.56 (0.18–0.92) | 0.003 | 0.758 |
| Overall phosphate management | 1.4 ± 1.0 | 1.5 ± 0.9 | 0.2 ±1.0 | - | 0.406 | 1.1 ± 1.0 | 1.6 ±0.9 | 0.5 ± 1.2 | - | 0.019 | 0.192 |
| Hyperphosphatemia consequences | 1.5 ± 1.0 | 1.9 ± 0.9 | 0.4 ± 0.8 | - | 0.016 | 1.6 ± 1.0 | 1.9 ±0.9 | 0.3 ± 0.9 | - | 0.032 | 0.884 |
| Phosphate binder | 2.4 ± 1.2 | 3.1 ± 1.0 | 0.8 ± 1.1 | - | <0.001 | 2.2 ± 1.1 | 2.9 ±1.1 | 0.7 ± 1.5 | - | 0.011 | 0.852 |
| Dietary phosphorus | 3.4 ±1.8 | 4.3 ± 1.5 | 0.9 ± 1.9 | - | 0.014 | 3.8 ± 1.8 | 4.5 ± 1.8 | 0.7 ± 2.5 | - | 0.118 | 0.742 |
| Responsibility | 1.0 ± 0.2 | 1.0 ± 0 | 0 ± 0.2 | - | 0.325 | 0.7 ± 0.5 | 0.9 ± 0.3 | 0.2 ± 0.6 | - | 0.018 | 0.042 |
| Medication Adherence | |||||||||||
| Total MMAS-4 score | 2.1 ± 1.1 | 3.2 ± 1.1 | 1.1 ± 1.2 | 0.90 (0.49–1.30) | <0.001 | 2.1 ± 1.1 | 2.9 ± 1.3 | 0.8 ± 1.5 | 0.50 (0.14–0.86) | 0.007 | 0.324 |
| Adherence | 4(12.1) | 20(60.6) | ↑ 16(48.5) | <0.001 a | 5(15.2) | 16 (48.5) | ↑ 11(33.3) | 0.001 a | 0.317 c | ||
| Non-adherence | 29(87.9) | 13(39.4) | 28(84.8) | 17(51.5) | |||||||
| Dietary Intakes | |||||||||||
| Energy (kcal/day) | 1623 ± 318 | 1433 ± 389 | −191 ± 306 | 0.62 (0.21–1.04) | 0.005 | 1609 ± 412 | 1392 ± 384 | −217 ± 274 | 0.79 (0.34–1.24) | 0.001 | 0.755 |
| Energy (kcal/kg/day) | 29.1 ± 6.3 | 25.6 ± 6.1 | −3.4 ± 5.8 | 0.60 (0.19–1.01) | 0.007 | 27.6 ± 7.7 | 23.9 ± 7.1 | −3.7 ± 4.9 | 0.75 (0.30–1.19) | 0.001 | 0.868 |
| Protein (g/day) | 60.9 ± 15.2 | 58.3 ± 17.2 | −2.6 ± 18.8 | 0.14 (0.00–0.52) | 0.492 | 60.2 ± 20.8 | 57.5 ± 17.2 | −2.7 ± 20.3 | 0.13 (0.00–0.52) | 0.520 | 0.989 |
| Protein (g/kg/day) | 1.1 ± 0.3 | 1.0 ± 0.3 | −0.0 ± 0.3 | 0.15 (0.00–0.52) | 0.471 | 1.0 ± 0.4 | 1.0 ± 0.3 | −0.0 ± 0.4 | 0.11 (0.00–0.49) | 0.601 | 0.929 |
| Phosphorus (mg/day) | 817 ± 280 | 629 ± 212 | −188 ± 161 | 1.17 (0.65–1.67) | <0.001 | 798 ± 267 | 572 ± 199 | −226 ± 193 | 1.17 (0.64–1.68) | <0.001 | 0.462 |
| PPR (mg/g) | 13.5 ± 3.8 | 11.6 ± 4.6 | −1.9 ± 3.1 | 0.62 (0.20–1.04) | 0.005 | 13.7 ± 3.7 | 10.0 ± 2.0 | −3.7 ± 3.5 | 1.07 (0.56–1.57) | <0.001 | 0.066 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teong, L.-F.; Khor, B.-H.; Ng, H.-M.; Sahathevan, S.; Purba, K.R.; Narayanan, S.S.; Gafor, A.H.A.; Goh, B.-L.; Bee, B.-C.; Yahya, R.; et al. Effectiveness of a Nutritional Mobile Application for Management of Hyperphosphatemia in Patients on Hemodialysis: A Multicenter Open-Label Randomized Clinical Trial. J. Pers. Med. 2022, 12, 961. https://doi.org/10.3390/jpm12060961
Teong L-F, Khor B-H, Ng H-M, Sahathevan S, Purba KR, Narayanan SS, Gafor AHA, Goh B-L, Bee B-C, Yahya R, et al. Effectiveness of a Nutritional Mobile Application for Management of Hyperphosphatemia in Patients on Hemodialysis: A Multicenter Open-Label Randomized Clinical Trial. Journal of Personalized Medicine. 2022; 12(6):961. https://doi.org/10.3390/jpm12060961
Chicago/Turabian StyleTeong, Lee-Fang, Ban-Hock Khor, Hi-Ming Ng, Sharmela Sahathevan, Kristo Radion Purba, Sreelakshmi Sankara Narayanan, Abdul Halim Abdul Gafor, Bak-Leong Goh, Boon-Cheak Bee, Rosnawati Yahya, and et al. 2022. "Effectiveness of a Nutritional Mobile Application for Management of Hyperphosphatemia in Patients on Hemodialysis: A Multicenter Open-Label Randomized Clinical Trial" Journal of Personalized Medicine 12, no. 6: 961. https://doi.org/10.3390/jpm12060961