Biomarkers of Myocardial Injury and Remodeling in Heart Failure
Abstract
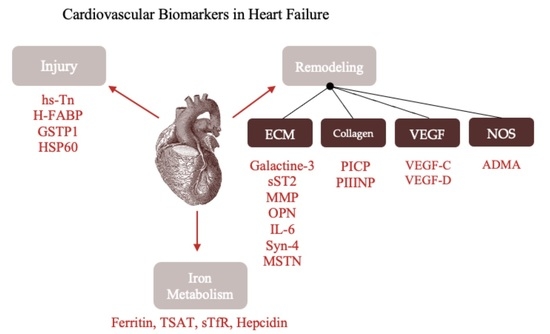
:1. Introduction
2. Biomarkers of Myocardial Remodeling
2.1. Cardiovascular Extracellular Matrix
2.1.1. Galectin-3
2.1.2. The Soluble Isoform of Suppression of Tumorigenicity 2
2.1.3. Matrix Metalloproteinases
2.1.4. Osteopontin
2.1.5. Interleukin-6
2.1.6. Syndecan-4
2.1.7. Myostatin
2.2. Collagen Metabolism
2.2.1. Procollagen Type I C-Terminal Propeptide
2.2.2. Procollagen Type III N-Terminal Propeptide
2.3. Vascular Endothelial Growth Factor
2.4. Nitric Oxidase Synthetases
Asymmetric Dimethylarginine
2.5. Clinical Perspectives
3. Biomarkers of Myocyte Injury
3.1. High-Sensitivity Troponins
3.2. Fatty Acid-Binding Proteins
3.3. Glutathione S-Transferase P1
3.4. Heat Shock Protein 60
3.5. Natriuretic Peptides
3.6. Clinical Perspectives
4. Biomarkers of Iron Metabolism
4.1. Ferritin
4.2. Transferin Saturation
4.3. Soluble Transferrin Receptor
4.4. Hepcidin
4.5. Clinical Perspectives
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.H.; Burnett, J.C. The natriuretic peptides in heart failure: Diagnostic and therapeutic potentials. Proc. Assoc. Am. Physicians 1999, 111, 406–416. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The Extracellular Matrix in Ischemic and Nonischemic Heart Failure. Circ. Res. 2019, 125, 117–146. [Google Scholar] [CrossRef] [PubMed]
- Dumic, J.; Dabelic, S.; Flögel, M. Galectin-3: An open-ended story. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 616–635. [Google Scholar] [CrossRef] [PubMed]
- De Boer, R.A.; Lok, D.J.A.; Jaarsma, T.; van der Meer, P.; Voors, A.A.; Hillege, H.L.; van Veldhuisen, D.J. Predictive Value of Plasma Galectin-3 Levels in Heart Failure with Reduced and Preserved Ejection Fraction. Ann. Med. 2011, 43, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Imran, T.F.; Shin, H.J.; Mathenge, N.; Wang, F.; Kim, B.; Joseph, J.; Gaziano, J.M.; Djoussé, L. Meta-Analysis of the Usefulness of Plasma Galectin-3 to Predict the Risk of Mortality in Patients with Heart Failure and in the General Population. Am. J. Cardiol. 2017, 119, 57–64. [Google Scholar] [CrossRef]
- Yu, L.; Ruifrok, W.P.; Meissner, M.; Bos, E.M.; van Goor, H.; Sanjabi, B.; van der Harst, P.; Pitt, B.; Goldstein, I.J.; Koerts, J.A.; et al. Genetic and Pharmacological Inhibition of Galectin-3 Prevents Cardiac Remodeling by Interfering with Myocardial Fibrogenesis. Circ. Heart Fail. 2013, 6, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-H.; D’Ambrosio, M.; Liao, T.-D.; Peng, H.; Rhaleb, N.-E.; Sharma, U.; André, S.; Gabius, H.-J.; Carretero, O.A. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am. J. Physiol. Circ. Physiol. 2009, 296, H404–H412. [Google Scholar] [CrossRef] [Green Version]
- Lotierzo, M.; Dupuy, A.M.; Kalmanovich, E.; Roubille, F.; Cristol, J.P. sST2 as a value-added biomarker in heart failure. Clin. Chim. Acta 2019, 501, 120–130. [Google Scholar] [CrossRef]
- Aimo, A.; Januzzi, J.L.; Bayes-Genis, A.; Vergaro, G.; Sciarrone, P.; Passino, C.; Emdin, M. Clinical and Prognostic Significance of sST2 in Heart Failure. J. Am. Coll. Cardiol. 2019, 74, 2193–2203. [Google Scholar] [CrossRef]
- Parikh, R.H.; Seliger, S.; Christenson, R.; Gottdiener, J.S.; Psaty, B.M.; Defilippi, C.R. Soluble ST2 for Prediction of Heart Failure and Cardiovascular Death in an Elderly, Community-Dwelling Population. J. Am. Heart Assoc. 2016, 5, e003188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medico-Economic Evaluation of Therapeutic Adaptation Guided by the Soluble Suppression of Tumorigenicity 2 (sST-2) Biomarker in the Management of Patients with Acute Heart Failure. Available online: https://clinicaltrials.gov/ct2/show/NCT04554277 (accessed on 6 May 2022).
- DeLeon-Pennell, K.Y.; Meschiari, C.A.; Jung, M.; Lindsey, M.L. Matrix Metalloproteinases in Myocardial Infarction and Heart Failure. Matrix metalloproteinases in myocardial infarction and heart failure. Prog. Mol. Biol. Transl. Sci. 2017, 147, 75–100. [Google Scholar] [PubMed] [Green Version]
- Halade, G.V.; Jin, Y.-F.; Lindsey, M.L. Matrix metalloproteinase (MMP)-9: A proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacol. Ther. 2013, 139, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, J.T.; Hallak, H.; Johnson, L.; Li, H.; O’Brien, P.M.; Sliskovic, D.R.; Bocan, T.M.A.; Coker, M.L.; Etoh, T.; Spinale, F.G. Matrix Metalloproteinase Inhibition Attenuates Left Ventricular Remodeling and Dysfunction in a Rat Model of Progressive Heart Failure. Circulation 2001, 103, 2303–2309. [Google Scholar] [CrossRef] [Green Version]
- Givvimani, S.; Tyagi, N.; Sen, U.; Mishra, P.K.; Qipshidze, N.; Munjal, C.; Vacek, J.C.; Abe, O.A.; Tyagi, S.C. MMP-2/TIMP-2/TIMP-4 versus MMP-9/TIMP-3 in transition from compensatory hypertrophy and angiogenesis to decompensatory heart failure. Arch. Physiol. Biochem. 2010, 116, 63–72. [Google Scholar] [CrossRef]
- Okamoto, H. Osteopontin and cardiovascular system. Mol. Cell. Biochem. 2006, 300, 1–7. [Google Scholar] [CrossRef]
- Singh, M.; Foster, C.R.; Dalal, S.; Singh, K. Role of osteopontin in heart failure associated with aging. Heart Fail. Rev. 2010, 15, 487–494. [Google Scholar] [CrossRef]
- Stawowy, P.; Blaschke, F.; Pfautsch, P.; Goetze, S.; Lippek, F.; Wollert-Wulf, B.; Fleck, E.; Graf, K. Increased myocardial expression of osteopontin in patients with advanced heart failure. Eur. J. Heart Fail. 2002, 4, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Soejima, H.; Irie, A.; Fukunaga, T.; Oe, Y.; Kojima, S.; Kaikita, K.; Kawano, H.; Sugiyama, S.; Yoshimura, M.; Kishikawa, H.; et al. Osteopontin Expression of Circulating T Cells and Plasma Osteopontin Levels are Increased in Relation to Severity of Heart Failure. Circ. J. 2007, 71, 1879–1884. [Google Scholar] [CrossRef] [Green Version]
- Schipper, M.E.; Scheenstra, M.R.; van Kuik, J.; van Wichen, D.F.; van der Weide, P.; Dullens, H.F.; Lahpor, J.; de Jonge, N.; de Weger, R.A. Osteopontin: A potential biomarker for heart failure and reverse remodeling after left ventricular assist device support. J. Heart Lung Transplant. 2011, 30, 805–810. [Google Scholar] [CrossRef]
- Li, J.; Yousefi, K.; Ding, W.; Singh, J.; Shehadeh, L.A. Osteopontin RNA aptamer can prevent and reverse pressure overload-induced heart failure. Cardiovasc. Res. 2017, 113, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Markousis-Mavrogenis, G.; Tromp, J.; Ouwerkerk, W.; Devalaraja, M.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.S.; van der Harst, P.; Lang, C.C.; et al. The clinical significance of interleukin-6 in heart failure: Results from the BIOSTAT-CHF study. Eur. J. Heart Fail. 2019, 21, 965–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chia, Y.C.; Kieneker, L.M.; van Hassel, G.; Binnenmars, S.H.; Nolte, I.M.; van Zanden, J.J.; van der Meer, P.; Navis, G.; Voors, A.A.; Bakker, S.J.L.; et al. Interleukin 6 and Development of Heart Failure with Preserved Ejection Fraction in the General Population. J. Am. Heart Assoc. 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Hanberg, J.S.; Rao, V.S.; Ahmad, T.; Chunara, Z.; Mahoney, D.; Jackson, K.; Jacoby, D.; Chen, M.; Wilson, F.P.; Tang, W.W.; et al. Inflammation and cardio-renal interactions in heart failure: A potential role for interleukin-6. Eur. J. Heart Fail. 2017, 20, 933–934. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Negishi, K.; Watanabe, A.; Arai, M.; Naganuma, F.; Ohyama, Y.; Kurabayashi, M. Serum syndecan-4 is a novel biomarker for patients with chronic heart failure. J. Cardiol. 2011, 57, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Xie, J.; Chen, J.; Li, R.; Wu, H.; Zhang, X.; Chen, Q.; Gu, R.; Xu, B. Syndecan-4 deficiency accelerates the transition from compensated hypertrophy to heart failure following pressure overload. Cardiovasc. Pathol. 2017, 28, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ouyang, P.; Zhang, Z.; Lai, W.; Xu, D. Changes and clinical significance of serum level of syndecan-4 protein in patients with chronic congestive heart failure. Chin. J. Cell. Mol. Immunol. 2013, 29, 866–869. [Google Scholar]
- Bielecka-Dabrowa, A.; von Haehling, S.; Aronow, W.S.; Ahmed, M.I.; Rysz, J.; Banach, M. Heart failure biomarkers in patients with dilated cardiomyopathy. Int. J. Cardiol. 2013, 168, 2404–2410. [Google Scholar] [CrossRef] [PubMed]
- Anker, M.S.; Von Haehling, S.; Springer, J. Blocking myostatin: Muscle mass equals muscle strength? J. Cachex Sarcopenia Muscle 2020, 11, 1396–1398. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; McMahon, C.D.; Matthews, K.G.; Devlin, G.P.; Elston, M.; Conaglen, J.V. Absence of Myostatin Improves Cardiac Function Following Myocardial Infarction. Heart Lung Circ. 2017, 27, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Liu, Z.; Luo, Y.; Chen, L.; Li, S.; Pan, Y.; Lei, X.; Wu, D.; Xu, D. Predictive value of serum myostatin for the severity and clinical outcome of heart failure. Eur. J. Intern. Med. 2019, 64, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Lijnen, P.J.; Maharani, T.; Finahari, N.; Prihadi, J.S. Serum Collagen Markers and Heart Failure. Cardiovasc. Hematol. Disord. Targets 2012, 12, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Querejeta, R.; López, B.; Gonzalez, A.; Sánchez, E.; Larman, M.; Ubago, J.L.M.; Díez, J. Increased Collagen Type I Synthesis in Patients with Heart Failure of Hypertensive Origin. Circulation 2004, 110, 1263–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demir, M.; Acartürk, E.; Inal, T.; Attila, G.; Dönmez, Y.; Avkaroğulları, M.; Çaylı, M. Procollagen type I carboxy-terminal peptide shows left ventricular hypertrophy and diastolic dysfunction in hypertensive patients. Cardiovasc. Pathol. 2007, 16, 69–74. [Google Scholar] [CrossRef]
- Yang, C.; Qiao, S.; Song, Y.; Liu, Y.; Tang, Y.; Deng, L.; Yuan, J.; Hu, F.; Yang, W. Procollagen type I carboxy-terminal propeptide (PICP) and MMP-2 are potential biomarkers of myocardial fibrosis in patients with hypertrophic cardiomyopathy. Cardiovasc. Pathol. 2019, 43, 107150. [Google Scholar] [CrossRef]
- Santanasto, A.J.; Cvejkus, R.K.; Wojczynski, M.K.; Marron, M.M.; Schupf, N.; Christensen, K.; Thyagarajan, B.; Zmuda, J.M. Circulating Procollagen Type III N-Terminal Peptide and Physical Function in Adults from the Long Life Family Study. J. Gerontol. A 2020, 76, 1273–1279. [Google Scholar] [CrossRef]
- Duprez, D.A.; Gross, M.D.; Kizer, J.R.; Ix, J.H.; Hundley, W.G.; Jacobs, D.R. Predictive Value of Collagen Biomarkers for Heart Failure with and without Preserved Ejection Fraction: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Heart Assoc. 2018, 7, e007885. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Taimeh, Z.; Loughran, J.; Birks, E.J.; Bolli, R. Vascular endothelial growth factor in heart failure. Nat. Rev. Cardiol. 2013, 10, 519–530. [Google Scholar] [CrossRef]
- Iguchi, M.; Wada, H.; Shinozaki, T.; Suzuki, M.; Ajiro, Y.; Matsuda, M.; Koike, A.; Koizumi, T.; Shimizu, M.; Ono, Y.; et al. Distinct association of VEGF-C and VEGF-D with prognosis in patients with chronic heart failure: The PREHOSP-CHF study. Eur. Heart J. 2021, 42 (Suppl. 1), ehab724-0868. [Google Scholar] [CrossRef]
- Víteček, J.; Lojek, A.; Valacchi, G.; Kubala, L. Arginine-Based Inhibitors of Nitric Oxide Synthase: Therapeutic Potential and Challenges. Mediat. Inflamm. 2012, 2012, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Xu, X.; Shang, R.; Chen, Y. Asymmetric dimethylarginine (ADMA) as an important risk factor for the increased cardiovascular diseases and heart failure in chronic kidney disease. Nitric Oxide 2018, 78, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Wang, Z.; Koeth, R.; Levison, B.; DelFraino, B.; Dzavik, V.; Griffith, O.W.; Hathaway, D.; Panza, J.A.; Nissen, S.E.; et al. Metabolic Profiling of Arginine and Nitric Oxide Pathways Predicts Hemodynamic Abnormalities and Mortality in Patients with Cardiogenic Shock After Acute Myocardial Infarction. Circulation 2007, 116, 2315–2324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böger, R.H.; Sullivan, L.M.; Schwedhelm, E.; Wang, T.J.; Maas, R.; Benjamin, E.J.; Schulze, F.; Xanthakis, V.; Benndorf, R.A.; Vasan, R.S. Plasma Asymmetric Dimethylarginine and Incidence of Cardiovascular Disease and Death in the Community. Circulation 2009, 119, 1592–1600. [Google Scholar] [CrossRef]
- Kielstein, J.T.; Bode-Böger, S.; Klein, G.; Graf, S.; Haller, H.; Fliser, D. Endogenous nitric oxide synthase inhibitors and renal perfusion in patients with heart failure. Eur. J. Clin. Investig. 2003, 33, 370–375. [Google Scholar] [CrossRef]
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the diagnosis and management of heart failure. Heart Fail. Rev. 2021, 27, 625–643. [Google Scholar] [CrossRef]
- Ibrahim, N.E.; Januzzi, J.L., Jr. Established and Emerging Roles of Biomarkers in Heart Failure. Circ. Res. 2018, 123, 614–629. [Google Scholar] [CrossRef]
- Felker, G.M.; Mentz, R.J.; Teerlink, J.R.; Voors, A.A.; Pang, P.; Ponikowski, P.; Greenberg, B.H.; Filippatos, G.; Davison, B.A.; Cotter, G.; et al. Serial high sensitivity cardiac troponin T measurement in acute heart failure: Insights from the RELAX-AHF study. Eur. J. Heart Fail. 2015, 17, 1262–1270. [Google Scholar] [CrossRef]
- Zymliński, R.; Sokolski, M.; Siwolowski, P.; Biegus, J.; Nawrocka, S.; Jankowska, E.; Todd, J.; Yerramilli, R.; Estis, J.; Banasiak, W.; et al. Elevated troponin I level assessed by a new high-sensitive assay and the risk of poor outcomes in patients with acute heart failure. Int. J. Cardiol. 2017, 230, 646–652. [Google Scholar] [CrossRef]
- Kelley, W.E.; Januzzi, J.L.; Christenson, R.H. Increases of Cardiac Troponin in Conditions other than Acute Coronary Syndrome and Heart Failure. Clin. Chem. 2009, 55, 2098–2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harjola, V.-P.; Mullens, W.; Banaszewski, M.; Bauersachs, J.; Brunner-La Rocca, H.P.; Chioncel, O.; Collins, S.P.; Doehner, W.; Filippatos, G.S.; Flammer, A.J.; et al. Organ dysfunction, injury and failure in acute heart failure: From pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2017, 19, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.; Melot, J.; Krinock, M.; Kumar, A.; Nadar, S.K.; Lip, G.Y.H. Heart-type fatty acid-binding protein: An overlooked cardiac biomarker. Ann. Med. 2020, 52, 444–461. [Google Scholar] [CrossRef] [PubMed]
- Savic-Radojevic, A.; Pljesa-Ercegovac, M.; Matic, M.; Simic, D.; Radovanovic, S.; Simic, T. Novel Biomarkers of Heart Failure. Adv. Clin. Chem. 2017, 79, 93–152. [Google Scholar] [CrossRef]
- Rezar, R.; Jirak, P.; Gschwandtner, M.; Derler, R.; Felder, T.K.; Haslinger, M.; Kopp, K.; Seelmaier, C.; Granitz, C.; Hoppe, U.C.; et al. Heart-Type Fatty Acid-Binding Protein (H-FABP) and Its Role as a Biomarker in Heart Failure: What Do We Know So Far? J. Clin. Med. 2020, 9, 164. [Google Scholar] [CrossRef] [Green Version]
- Andrukhova, O.; Salama, M.; Rosenhek, R.; Gmeiner, M.; Perkmann, T.; Steindl, J.; Aharinejad, S. Serum Glutathione S-Transferase P1 1 in Prediction of Cardiac Function. J. Card. Fail. 2012, 18, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Farré, A.L.; Casado, S. Heart Failure, Redox Alterations, and Endothelial Dysfunction. Hypertension 2001, 38, 1400–1405. [Google Scholar] [CrossRef]
- Bonanad, C.; Núñez, J.; Sanchis, J.; Bodí, V.; Chaustre, F.; Chillet, M.; Minana, G.; Forteza, M.J.; Palau, P.; Nunez, E.; et al. Serum Heat Shock Protein 60 in Acute Heart Failure: A New Biomarker? Congest. Heart Fail. 2012, 19, 6–10. [Google Scholar] [CrossRef]
- Lewthwaite, J.; Owen, N.; Coates, A.; Henderson, B.; Steptoe, A. Circulating Human Heat Shock Protein 60 in the Plasma of British Civil Servants. Circulation 2002, 106, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Niizeki, T.; Takeishi, Y.; Watanabe, T.; Nitobe, J.; Miyashita, T.; Miyamoto, T.; Kitahara, T.; Suzuki, S.; Sasaki, T.; Bilim, O.; et al. Relation of Serum Heat Shock Protein 60 Level to Severity and Prognosis in Chronic Heart Failure Secondary to Ischemic or Idiopathic Dilated Cardiomyopathy. Am. J. Cardiol. 2008, 102, 606–610. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Butler, J.; Fombu, E.; Maisel, A.; McCague, K.; Piña, I.L.; Prescott, M.F.; Riebman, J.B.; Solomon, S. Rationale and methods of the Prospective Study of Biomarkers, Symptom Improvement, and Ventricular Remodeling During Sacubitril/Valsartan Therapy for Heart Failure (PROVE-HF). Am. Heart J. 2018, 199, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Loncar, G.; Obradovic, D.; Thiele, H.; von Haehling, S.; Lainscak, M. Iron deficiency in heart failure. ESC Heart Fail. 2021, 8, 2368–2379. [Google Scholar] [CrossRef] [PubMed]
- Ghafourian, K.; Shapiro, J.S.; Goodman, L.; Ardehali, H. Iron and Heart Failure. JACC Basic Transl. Sci. 2020, 5, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Von Haehling, S.; Jankowska, E.; van Veldhuisen, D.J.; Ponikowski, P.; Anker, S.D. Iron deficiency and cardiovascular disease. Nat. Rev. Cardiol. 2015, 12, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Łęcka, M.; Słomka, A.; Albrecht, K.; Żekanowska, E.; Romiszewski, M.; Styczyński, J. Unbalance in Iron Metabolism in Childhood Leukemia Converges with Treatment Intensity: Biochemical and Clinical Analysis. Cancers 2021, 13, 3029. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.; Damy, T.; Doehner, W.; Lam, C.S.; Sindone, A.; van der Meer, P.; Cohen-Solal, A.; Kindermann, I.; Manito, N.; Pfister, O.; et al. Screening, diagnosis and treatment of iron deficiency in chronic heart failure: Putting the 2016 European Society of Cardiology heart failure guidelines into clinical practice. Eur. J. Heart Fail. 2018, 20, 1664–1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankowska, E.A.; Kasztura, M.; Sokolski, M.; Bronisz, M.; Nawrocka, S.; Oleśkowska-Florek, W.; Zymlinski, R.; Biegus, J.; Siwołowski, P.; Banasiak, W.; et al. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur. Heart J. 2014, 35, 2468–2476. [Google Scholar] [CrossRef] [PubMed]
- Beverborg, N.G.; Klip, I.T.; Meijers, W.C.; Voors, A.A.; Vegter, E.L.; van der Wal, H.H.; Swinkels, D.W.; van Pelt, J.; Mulder, A.B.; Bulstra, S.K.; et al. Definition of Iron Deficiency Based on the Gold Standard of Bone Marrow Iron Staining in Heart Failure Patients. Circ. Heart Fail. 2018, 11, e004519. [Google Scholar] [CrossRef]
- Braga, F.; Infusino, I.; Dolci, A.; Panteghini, M. Soluble transferrin receptor in complicated anemia. Clin. Chim. Acta 2014, 431, 143–147. [Google Scholar] [CrossRef]
- Sierpinski, R.; Josiak, K.; Suchocki, T.; Wojtas-Polc, K.; Mazur, G.; Butrym, A.; Rozentryt, P.; Meer, P.; Comin-Colet, J.; Haehling, S.; et al. High soluble transferrin receptor in patients with heart failure: A measure of iron deficiency and a strong predictor of mortality. Eur. J. Heart Fail. 2021, 23, 919–932. [Google Scholar] [CrossRef]
- Biegus, J.; Zymliński, R.; Sokolski, M.; Jankowska, E.A.; Banasiak, W.; Ponikowski, P. Elevated lactate in acute heart failure patients with intracellular iron deficiency as identifier of poor outcome. Kardiol. Pol. 2019, 77, 347–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Biomarker | Clinical Significance |
|---|---|
| Galectin-3 | Prediction of HF development; Correlation with cardiovascular and all-cause mortality |
| The soluble isoform of suppression of tumorigenicity 2 (sST2) | Prediction of HF development; Correlation with cardiovascular morality |
| Matrix metalloproteinases (MMP) | Prediction of post-MI HF development; Correlation with post-MI mortality |
| Matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases balance (MMP/TIMP) | Prediction of HF development |
| Osteopontin (OPN) | Assessment of HF severity; Association with reverse cardiac remodeling |
| Interleukin-6 (IL-6) | Prediction of HFpEF development; Association with renal function in HF; Prediction of unscheduled HF hospitalization; Correlation with all-cause, cardiovascular and non-cardiovascular mortality |
| Syndecan-4 (Syn-4) | Correlation with left ventricle geometrical parameters in CHF |
| Myostatin (MSTN) | Assessment of HF severity; Prediction of CHF development; Correlation with CHF survival rate and number of rehospitalizations |
| Procollagen Type-1 C-terminal propeptide (PICP) | Prediction of hypertensive origin HF development; Correlation with ventricular hypertrophy, diastolic function and cardiac fibrosis |
| Procollagen type III N-terminal propeptide (PIIINP) | Prediction of HFpEF development; Correlation with all-cause and cardiovascular mortality |
| Acute HF | Chronic HF |
|---|---|
| Additional value of combining with NT-proBNP to rule out the diagnosis of AHF [48] Elevated serum levels as a predictive marker of acute kidney injury [49] Additional value of either elevated serum levels or in combination with troponin I and NT-proBNP for prognostic assessment [48,49] | In HF with preserved ejection fraction, it is an independent predictor of cardiovascular events [2] |
| Application | Biomarkers |
|---|---|
| Measurement of functional and storage iron pools | Iron Transferrin Total iron-binding capacity Ferritin Non-transferrin-bound iron Labile plasma iron levels |
| Assessment of proteins regulating iron absorption and release from tissue stores | Serum hepcidin Soluble hemojuvelin Soluble ferroportin-1 |
| Assessment of proteins regulating the erythropoietic activity of bone marrow | Erythroferrone Soluble transferrin receptor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponikowska, B.; Iwanek, G.; Zdanowicz, A.; Urban, S.; Zymliński, R.; Ponikowski, P.; Biegus, J. Biomarkers of Myocardial Injury and Remodeling in Heart Failure. J. Pers. Med. 2022, 12, 799. https://doi.org/10.3390/jpm12050799
Ponikowska B, Iwanek G, Zdanowicz A, Urban S, Zymliński R, Ponikowski P, Biegus J. Biomarkers of Myocardial Injury and Remodeling in Heart Failure. Journal of Personalized Medicine. 2022; 12(5):799. https://doi.org/10.3390/jpm12050799
Chicago/Turabian StylePonikowska, Barbara, Gracjan Iwanek, Agata Zdanowicz, Szymon Urban, Robert Zymliński, Piotr Ponikowski, and Jan Biegus. 2022. "Biomarkers of Myocardial Injury and Remodeling in Heart Failure" Journal of Personalized Medicine 12, no. 5: 799. https://doi.org/10.3390/jpm12050799