Assessment of Dynamic Changes in Stressed Volume and Venous Return during Hyperdynamic Septic Shock
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extrapolation Model of Calculation of Stressed Volume
2.2. Experimental Model
2.2.1. Ethics Approval
2.2.2. Study Objectives
2.2.3. Origin and Source of the Animals
2.2.4. Animal Preparation
2.2.5. Preparation of Bacterial Suspensions
2.2.6. Experimental Procedure
2.2.7. Calculation of Baseline Mean Circulatory Filling Pressure Analogue and Related Variables
2.2.8. Analysis of the Dynamic Changes in Stressed Volume during Progression of Septic Shock
2.2.9. Calculation of Mean Circulatory Filling Pressure during Cardiac Arrest
2.2.10. Statistical Analysis
3. Results
3.1. Progression of Sepsis and Septic Shock
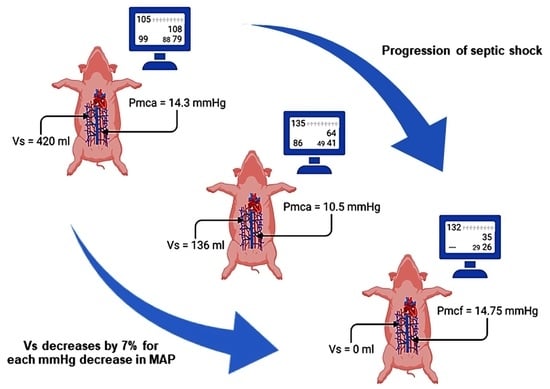
3.2. Dynamic Changes in Stressed Volume during Progression of Septic Shock
3.3. Changes in Mean Circulatory Filling Pressure Analogue and Other Determinants of Venous Return during Septic Shock
3.4. Effects of Fluid Challenges and Noradrenaline on Determinants of Venous Return
3.5. Measurement of Mean Circulatory Filling Pressure after Cardiac Arrest
4. Discussion
4.1. Estimation and Dynamic Changes in Stressed Volume
4.2. Conceptual Approach and Characteristics of Rest Volume
4.3. Dynamic Changes in Mean Circulatory Filling Pressure and Other Determinants of Venous Return
4.4. Clinical Implications
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gelman, S.; Bigatello, L. The physiologic basis for goal-directed hemodynamic and fluid therapy: The pivotal role of the venous circulation. Can. J. Anaesth. 2018, 65, 294–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, S.; Chang, C.; Dellenback, R.J.; Usami, S.; Gregersen, M.I. Hemodynamic changes in endotoxin shock. Am. J. Physiol. 1966, 210, 1401–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinsky, M.R.; Matuschak, G.M. Cardiovascular determinants of the hemodynamic response to acute endotoxemia in the dog. J. Crit. Care 1986, 1, 18–31. [Google Scholar] [CrossRef]
- Teule, G.J.; den Hollander, W.; Bronsveld, W.; Koopman, P.A.; Bezemer, P.D.; Heidendal, G.A.; Thijs, L.G. Effect of volume loading and dopamine on hemodynamics and red-cell redistribution in canine endotoxin shock. Circ. Shock. 1983, 10, 41–50. [Google Scholar] [PubMed]
- Stephan, F.; Novara, A.; Tournier, B.; Maillet, J.M.; London, G.M.; Safar, M.E.; Fagon, J.Y. Determination of total effective vascular compliance in patients with sepsis syndrome. Am. J. Respir. Crit. Care Med. 1998, 157, 50–56. [Google Scholar] [CrossRef]
- Funk, D.J.; Jacobsohn, E.; Kumar, A. Role of the venous return in critical illness and shock: Part II-shock and mechanical ventilation. Crit. Care Med. 2013, 41, 573–579. [Google Scholar] [CrossRef]
- Marik, P.E.; Weinmann, M. Optimizing fluid therapy in shock. Curr. Opin. Crit. Care 2019, 25, 246–251. [Google Scholar] [CrossRef]
- Marik, P.E.; Farkas, J.D. The Changing Paradigm of Sepsis: Early Diagnosis, Early Antibiotics, Early Pressors, and Early Adjuvant Treatment. Crit. Care Med. 2018, 46, 1690–1692. [Google Scholar] [CrossRef]
- Chalkias, A.; Spyropoulos, V.; Koutsovasilis, A.; Papalois, A.; Kouskouni, E.; Xanthos, T. Cardiopulmonary Arrest and Resuscitation in Severe Sepsis and Septic Shock: A Research Model. Shock 2015, 43, 285–291. [Google Scholar] [CrossRef]
- Xanthos, T.; Lelovas, P.; Vlachos, I.; Tsirikos-Karapanos, N.; Kouskouni, E.; Perrea, D.; Dontas, I. Cardiopulmonary arrest and resuscitation in Landrace/Large White swine: A research model. Lab. Anim. 2007, 41, 353–362. [Google Scholar] [CrossRef]
- Gelman, S. Venous Circulation: A Few Challenging Concepts in Goal-Directed Hemodynamic Therapy (GDHT). In Perioperative Fluid Management; Farag, E., Kurz, A., Troianos, C., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 365–385. [Google Scholar]
- Rothe, C.F. Mean circulatory filling pressure: Its meaning and measurement. J. Appl. Physiol. 1985, 1993, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Gelman, S. Venous function and central venous pressure: A physiologic story. Anesthesiology 2008, 108, 735–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magder, S. Volume and its relationship to cardiac output and venous return. Crit. Care 2016, 20, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalkias, A.; Koutsovasilis, A.; Laou, E.; Papalois, A.; Xanthos, T. Measurement of mean systemic filling pressure after severe hemorrhagic shock in swine anesthetized with propofol-based total intravenous anesthesia: Implications for vasopressor-free resuscitation. Acute. Crit. Care 2020, 35, 93–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magder, S.; De Varennes, B. Clinical death and the measurement of stressed vascular volume. Crit. Care Med. 1998, 26, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Gelman, S.; Mushlin, P.S. Catecholamine-induced changes in the splanchnic circulation affecting systemic hemodynamics. Anesthesiology 2004, 100, 434–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Centre for the Replacement, Refinement & Reduction of Animals in Research. The 3Rs. Available online: https://www.nc3rs.org.uk/the-3rs (accessed on 10 February 2022).
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Osuchowski, M.F.; Ayala, A.; Bahrami, S.; Bauer, M.; Boros, M.; Cavaillon, J.M.; Chaudry, I.H.; Coopersmith, C.M.; Deutschman, C.; Drechsler, S.; et al. Minimum quality threshold in pre-clinical sepsis studies (MQTiPSS): An international expert consensus initiative for improvement of animal modeling in sepsis. Intensive Care Med. Exp. 2018, 6, 26. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Swindle, M.M.; Vogler, G.A.; Fulton, L.K.; Marini, R.P.; Popilskis, S. Preanaesthesia, anesthesia, analgesia and euthanasia. In Laboratory Animal Medicine, 2nd ed.; Fox, J.G., Anderson, L.C., Loew, F.M., Quimby, F.W., Eds.; Academic Press: New York, NY, USA, 2002; pp. 955–1003. [Google Scholar]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef]
- Wodack, K.H.; Graessler, M.F.; Nishimoto, S.A.; Behem, C.R.; Pinnschmidt, H.O.; Punke, M.A.; Monge-García, M.I.; Trepte, C.J.C.; Reuter, D.A. Assessment of central hemodynamic effects of phenylephrine: An animal experiment. J. Clin. Monit. Comput. 2019, 33, 377–384. [Google Scholar] [CrossRef]
- Parkin, G.; Wright, C.; Bellomo, R.; Boyce, N. Use of a mean systemic filling pressure analogue during the closed-loop control of fluid replacement in continuous hemodiafiltration. J. Crit. Care 1994, 9, 124–133. [Google Scholar] [CrossRef]
- Parkin, W.G. Volume state control—A new approach. Crit. Care Resusc. 1999, 1, 311–321. [Google Scholar] [PubMed]
- Parkin, W.G.; Leaning, M.S. Therapeutic control of the circulation. J. Clin. Monit. Comput. 2008, 22, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, V.A.; Mudaliar, Y.; Gopalakrishnan, M.; Horton, M.D.; Killick, C.J.; Parkin, W.G.; Playford, H.R.; Raper, R.F. Computer based haemodynamic guidance system is effective and safe in management of postoperative cardiac surgery patients. Anaesth. Intensive. Care 2011, 39, 191–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meijs, L.P.B.; van Houte, J.; Conjaerts, B.C.M.; Bindels, A.J.G.H.; Bouwman, A.; Houterman, S.; Bakker, J. Clinical validation of a computerized algorithm to determine mean systemic filling pressure. J. Clin. Monit. Comput. 2022, 36, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Wijnberge, M.; Sindhunata, D.P.; Pinsky, M.R.; Vlaar, A.P.; Ouweneel, E.; Jansen, J.R.; Veelo, D.P.; Geerts, B.F. Estimating mean circulatory filling pressure in clinical practice: A systematic review comparing three bedside methods in the critically ill. Ann. Intensive. Care 2018, 8, 73. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.M.; Ogundele, O.; Pike, F.; Pinsky, M.R. Effect of acute endotoxemia on analog estimates of mean systemic pressure. J. Crit. Care 2013, 28, 880.e9–880.e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, D.; Moller, P.W.; Takala, J. Reply to “Letter to the editor: Why persist in the fallacy that mean systemic pressure drives venous return?”. Am. J. Physiol. Heart. Circ. Physiol. 2016, 311, H1336–H1337. [Google Scholar] [CrossRef] [Green Version]
- Berger, D.; Moller, P.W.; Weber, A.; Bloch, A.; Bloechlinger, S.; Haenggi, M.; Sondergaard, S.; Jakob, S.M.; Magder, S.; Takala, J. Effect of PEEP, blood volume, and inspiratory hold maneuvers on venous return. Am. J. Physiol. Heart. Circ. Physiol. 2016, 311, H794–H806. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Yuan, S.; Long, Y.; Liu, D.; Zhou, X.; Ince, C. Effect of norepinephrine challenge on cardiovascular determinants assessed using a mathematical model in septic shock: A physiological study. Ann. Transl. Med. 2021, 9, 561. [Google Scholar] [CrossRef]
- Guyton, A.C.; Polizo, D.; Armstrong, G.G. Mean circulatory filling pressure measured immediately after cessation of heart pumping. Am. J. Physiol. 1954, 179, 261–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalkias, A.; Xanthos, T. Pathophysiology and pathogenesis of post-resuscitation myocardial stunning. Heart. Fail. Rev. 2012, 17, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, R.I.; Zborowska-Sluis, D.; Tenaschuk, B. Measurement of mean circulatory filling pressure and vascular compliance in domestic pigs. Am. J. Physiol. 1990, 258, H1925–H1932. [Google Scholar] [CrossRef] [PubMed]
- Schipke, J.D.; Heusch, G.; Sanii, A.P.; Gams, E.; Winter, J. Static filling pressure in patients during induced ventricular fibrillation. Am. J. Physiol. Heart. Circ. Physiol. 2003, 285, H2510–H2515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fessler, H.E.; Brower, R.G.; Wise, R.A.; Permutt, S. Effects of positive end-expiratory pressure on the gradient for venous return. Am. Rev. Respir. Dis. 1991, 143, 19–24. [Google Scholar] [CrossRef]
- Jellinek, H.; Krenn, H.; Oczenski, W.; Veit, F.; Schwarz, S.; Fitzgerald, R.D. Influence of positive airway pressure on the pressure gradient for venous return in humans. J. Appl. Physiol. 2000, 88, 926–932. [Google Scholar] [CrossRef]
- Pironet, A.; Desaive, T.; Geoffrey Chase, J.; Morimont, P.; Dauby, P.C. Model-based computation of total stressed blood volume from a preload reduction manoeuvre. Math. Biosci. 2015, 265, 28–39. [Google Scholar] [CrossRef] [Green Version]
- Drees, J.A.; Rothe, C.F. Reflex venoconstriction and capacity vessel pressure-volume relationships in dogs. Circ. Res. 1974, 34, 360–373. [Google Scholar] [CrossRef] [Green Version]
- Rothe, C.F.; Drees, J.A. Vascular capacitance and fluid shifts in dogs during prolonged hemorrhagic hypotension. Circ. Res. 1976, 38, 347–356. [Google Scholar] [CrossRef] [Green Version]
- Ogilvie, R.I.; Zborowska-Sluis, D. Effect of chronic rapid ventricular pacing on total vascular capacitance. Circulation 1992, 85, 1524–1530. [Google Scholar] [CrossRef] [Green Version]
- Maas, J.J.; Pinsky, M.R.; Aarts, L.P.; Jansen, J.R. Bedside assessment of total systemic vascular compliance, stressed volume, and cardiac function curves in intensive care unit patients. Anesth. Analg. 2012, 115, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Maas, J.J.; Geerts, B.F.; van den Berg, P.C.; Pinsky, M.R.; Jansen, J.R. Assessment of venous return curve and mean systemic filling pressure in postoperative cardiac surgery patients. Crit. Care Med. 2009, 37, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.; Davidson, S.; Chase, J.G.; Knopp, J.L.; Zhou, T.; Desaive, T. Patient-Specific Monitoring and Trend Analysis of Model-Based Markers of Fluid Responsiveness in Sepsis: A Proof-of-Concept Animal Study. Ann. Biomed. Eng. 2020, 48, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Uemura, K.; Kawada, T.; Zheng, C.; Li, M.; Sugimachi, M. Computer-controlled closed-loop drug infusion system for automated hemodynamic resuscitation in endotoxin-induced shock. BMC Anesthesiol. 2017, 17, 145. [Google Scholar] [CrossRef] [Green Version]
- Kontouli, Z.; Staikou, C.; Iacovidou, N.; Mamais, I.; Kouskouni, E.; Papalois, A.; Papapanagiotou, P.; Gulati, A.; Chalkias, A.; Xanthos, T. Resuscitation with centhaquin and 6% hydroxyethyl starch 130/0.4 improves survival in a swine model of hemorrhagic shock: A randomized experimental study. Eur. J. Trauma. Emerg. Surg. 2019, 45, 1077–1085. [Google Scholar] [CrossRef]
- Chalkias, A.; Spyropoulos, V.; Georgiou, G.; Laou, E.; Koutsovasilis, A.; Pantazopoulos, I.; Kolonia, K.; Vrakas, S.; Papalois, A.; Demeridou, S.; et al. Baseline Values and Kinetics of IL-6, Procalcitonin, and TNF-α in Landrace-Large White Swine Anesthetized with Propofol-Based Total Intravenous Anesthesia. BioMed Res. Int. 2021, 2021, 6672573. [Google Scholar] [CrossRef]
- Brengelmann, G.L. Venous return and the physical connection between distribution of segmental pressures and volumes. Am. J. Physiol. Heart. Circ. Physiol. 2019, 317, H939–H953. [Google Scholar] [CrossRef]
- Girling, F. Critical closing pressure and venous pressure. Am. J. Physiol. 1952, 171, 204–207. [Google Scholar] [CrossRef] [Green Version]
- Thiele, R.H.; Nemergut, E.C.; Lynch, C., 3rd. The physiologic implications of isolated alpha(1) adrenergic stimulation. Anesth. Analg. 2011, 113, 284–296. [Google Scholar] [CrossRef] [Green Version]
- Bressack, M.A.; Morton, N.S.; Hortop, J. Group B streptococcal sepsis in the piglet: Effects of fluid therapy on venous return, organ edema, and organ blood flow. Circ. Res. 1987, 61, 659–669. [Google Scholar] [CrossRef] [Green Version]
- Ayuse, T.; Brienza, N.; Revelly, J.P.; O’Donnell, C.P.; Boitnott, J.K.; Robotham, J.L. Alternations in liver hemodynamics in an intact porcine model of endotoxin shock. Am. J. Physiol. 1995, 268, H1106–H1114. [Google Scholar] [CrossRef]
- Thiele, R.H.; Nemergut, E.C.; Lynch, C., 3rd. The clinical implications of isolated alpha1 adrenergic stimulation. Anesth. Analg. 2011, 113, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Persichini, R.; Silva, S.; Teboul, J.L.; Jozwiak, M.; Chemla, D.; Richard, C.; Monnet, X. Effects of norepinephrine on mean systemic pressure and venous return in human septic shock. Crit. Care Med. 2012, 40, 3146–3153. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef]
- Åneman, A.; Wilander, P.; Zoerner, F.; Lipcsey, M.; Chew, M.S. Vasopressor Responsiveness Beyond Arterial Pressure: A Conceptual Systematic Review Using Venous Return Physiology. Shock. 2021, 56, 352–359. [Google Scholar]
- Guarracino, F.; Bertini, P.; Pinsky, M.R. Cardiovascular determinants of resuscitation from sepsis and septic shock. Crit. Care 2019, 23, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blain, C.M.; Anderson, T.O.; Pietras, R.J.; Gunnar, R.M. Immediate hemodynamic effects of gram-negative vs gram-positive bacteremia in man. Arch. Intern. Med. 1970, 126, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Haery, C.; Parrillo, J.E. Myocardial dysfunction in septic shock: Part I. Clinical manifestation of cardiovascular dysfunction. J. Cardiothorac. Vasc. Anesth. 2001, 15, 364–376. [Google Scholar] [CrossRef]
- MacLean, L.D.; Mulligan, W.G.; McLean, A.P.; Duff, J.H. Patterns of septic shock in man—A detailed study of 56 patients. Ann. Surg. 1967, 166, 543–562. [Google Scholar] [CrossRef]
- Weil, M.H.; Nishjima, H. Cardiac output in bacterial shock. Am. J. Med. 1978, 64, 920–922. [Google Scholar] [CrossRef]
- Chien, S.; Dellenback, R.J.; Usami, S.; Treitel, K.; Chang, C.; Gregersen, M.I. Blood volume and its distribution in endotoxin shock. Am. J. Physiol. 1966, 210, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Teule, G.J.; Kester, A.D.; Bezemer, P.D.; Thijs, L.J.; Heidendal, G.A. Hepatic trapping of red cells in canine endotoxin shock: A variable phenomenon after splenectomy. Cardiovasc. Res. 1985, 19, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Repessé, X.; Charron, C.; Fink, J.; Beauchet, A.; Deleu, F.; Slama, M.; Belliard, G.; Vieillard-Baron, A. Value and determinants of the mean systemic filling pressure in critically ill patients. Am. J. Physiol. Heart. Circ. Physiol. 2015, 309, H1003–H1007. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, M.R.; Payen, D. Functional hemodynamic monitoring. Crit. Care 2005, 9, 566–572. [Google Scholar] [CrossRef] [Green Version]
- Toscani, L.; Aya, H.D.; Antonakaki, D.; Bastoni, D.; Watson, X.; Arulkumaran, N.; Rhodes, A.; Cecconi, M. What is the impact of the fluid challenge technique on diagnosis of fluid responsiveness? A systematic review and meta-analysis. Crit. Care 2017, 21, 207. [Google Scholar] [CrossRef] [Green Version]
- Guarracino, F.; Bertini, P.; Pinsky, M.R. Heterogeneity of cardiovascular response to standardized sepsis resuscitation. Crit. Care 2020, 24, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, G.; Ospina-Tascon, G.A.; Damiani, L.P.; Estenssoro, E.; Dubin, A.; Hurtado, J.; Friedman, G.; Castro, R.; Alegria, L.; Teboul, J.L.; et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality among Patients with Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA. 2019, 321, 654–664. [Google Scholar] [CrossRef]
- Monnet, X.; Marik, P.E.; Teboul, J.L. Prediction of fluid responsiveness: An update. Ann. Intensive. Care 2016, 6, 111. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.L.; Cecconi, M.; De Backer, D. The fluid challenge. Crit. Care 2020, 24, 703. [Google Scholar] [CrossRef]
- Shi, R.; Monnet, X.; Teboul, J.L. Parameters of fluid responsiveness. Curr. Opin. Crit. Care 2020, 26, 319–326. [Google Scholar] [CrossRef]
- Vignon, P.; Repesse, X.; Begot, E.; Leger, J.; Jacob, C.; Bouferrache, K.; Slama, M.; Prat, G.; Vieillard-Baron, A. Comparison of echocardiographic indices used to predict fluid responsiveness in ventilated patients. Am. J. Respir. Crit. Care Med. 2017, 195, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Tascon, G.A.; Teboul, J.L.; Hernandez, G.; Alvarez, I.; Sanchez-Ortiz, A.I.; Calderon-Tapia, L.E.; Manzano-Nunez, R.; Quinones, E.; Madrinan-Navia, H.J.; Ruiz, J.E.; et al. Diastolic shock index and clinical outcomes in patients with septic shock. Ann. Intensive. Care 2020, 10, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colon Hidalgo, D.; Patel, J.; Masic, D.; Park, D.; Rech, M.A. Delayed vasopressor initiation is associated with increased mortality in patients with septic shock. J. Crit. Care 2020, 55, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Tascon, G.A.; Hernandez, G.; Alvarez, I.; Calderon-Tapia, L.E.; Manzano-Nunez, R.; Sanchez-Ortiz, A.I.; Quinones, E.; Ruiz-Yucuma, J.E.; Aldana, J.L.; Teboul, J.L.; et al. Effects of very early start of norepinephrine in patients with septic shock: A propensity score-based analysis. Crit. Care 2020, 24, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Permpikul, C.; Tongyoo, S.; Viarasilpa, T.; Trainarongsakul, T.; Chakorn, T.; Udompanturak, S. Early use of norepinephrine in septic shock resuscitation (CENSER): A randomized trial. Am. J. Respir. Crit. Care Med. 2019, 199, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, H.; Zhang, D. Timing of norepinephrine initiation in patients with septic shock: A systematic review and meta-analysis. Crit. Care 2020, 24, 488. [Google Scholar] [CrossRef]
- Pecchiari, M.; Pontikis, K.; Alevrakis, E.; Vasileiadis, I.; Kompoti, M.; Koutsoukou, A. Cardiovascular Responses During Sepsis. Compr. Physiol. 2021, 11, 1605–1652. [Google Scholar]
Short Biography of Author




| Baseline | 1 h | 2 h | 3 h | 4 h | 5 h | 6 h | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Heart rate (beat·min−1) | 127.2 (14.23) | 137.4 (12.19) | 137 (19.09) | 134.6 (18.63) | 142.7 (18.03) | 123.5 (14.94) | 129.1 (15.56) | 0.135 |
| MAP (mmHg) | 88.4 (20.94) | 78.8 (20.35) | 59.6 (13.50) | 48.6 (13.81) | 48.6 (15.94) | 42.7 (12.26) | 33.2 (3.36) | <0.001 |
| CO (L·min−1) | 6.4 (0.34) | 6.9 (0.22) | 7.4 (0.25) | 8 (0.11) | 8.6 (0.19) | 8.7 (0.41) | 10.1 (0.53) | <0.001 |
| SVR (dynes·sec·cm−5) | 1012.7 (61.24) | 827.5 (42.79) | 585.3 (18.06) | 443.4 (11.99) | 416.2 (14.16) | 346.2 (16.98) | 244.6 (17.78) | <0.001 |
| PRA (mmHg) | 7.3 (1.16) | 6.6 (0.84) | 5.5 (0.71) | 4.1 (0.74) | 4 (0.67) | 4.9 (0.32) | 2.4 (0.52) | <0.001 |
| Pmca (mmHg) | 14.3 (1.23) | 13.5 (0.85) | 11.9 (0.74) | 10.5 (0.71) | 10.8 (0.64) | 11.5 (0.38) | 9.5 (0.57) | <0.001 |
| PGVR (mmHg) | 6.9 (0.16) | 6.9 (0.11) | 6.4 (0.18) | 6.4 (0.08) | 6.8 (0.12) | 6.6 (0.24) | 7.1 (0.3) | 0.934 |
| RVR (mmHg·min·L−1) | 1.1 (0.03) | 1 (0.02) | 0.87 (0.01) | 0.8 (0.01) | 0.79 (0.01) | 0.75 (0.01) | 0.7 (0.01) | <0.001 |
| Eh | 0.49 (0.04) | 0.52 (0.03) | 0.54 (0.03) | 0.61 (0.04) | 0.63 (0.04) | 0.57 (0.02) | 0.75 (0.04) | <0.001 |
| Vs (mL) | 420 | 350 | 214 | 136 | 136 | 93 | ≈0 | <0.001 |
| 2 h (100 mL) | 3 h (300 mL) | 4 h (200 mL) | |||||
|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | p-Value | |
| Heart rate (beat·min−1) | 140 (15) | 126 (7) | 138 (4) | 137 (6) | 148 (12) | 148 (9) | 1 |
| MAP (mmHg) | 61 (11) | 64 (6) | 46 (5) | 46 (6) | 45 (7) | 45 (4) | 1 |
| CO (L·min−1) | 7.1 (2) | 7.3 (2) | 7.9 (2) | 8 (2) | 8.5 (2) | 8.6 (2) | 0.79 |
| SVR (dynes·sec·cm−5) | 629 (14) | 642 (8) | 424 (16) | 420 (11) | 386 (24) | 381 (17) | 0.98 |
| PRA (mmHg) | 5.2 (0.2) | 5.4 (0.5) | 4.1 (0.3) | 4 (0.2) | 4 (0.4) | 4 (0.5) | 1 |
| Pmca (mmHg) | 11.6 (0.4) | 12 (0.3) | 10.4 (0.8) | 10.3 (0.2) | 10.6 (0.3) | 10.6 (0.3) | 1 |
| PGVR (mmHg) | 6.4 (0.5) | 6.6 (0.2) | 6.3 (0.2) | 6.3 (0.3) | 6.6 (0.3) | 6.6 (0.1) | 1 |
| RVR (mmHg·min·L−1) | 0.9 (0.1) | 0.9 (0.2) | 0.8 (0.2) | 0.8 (0.3) | 0.8 (0.2) | 0.8 (0.2) | 1 |
| Eh | 0.55 (0.02) | 0.55 (0.03) | 0.61 (0.01) | 0.61 (0.01) | 0.62 (0.01) | 0.62 (0.01) | 1 |
| Vs (mL) | 221 | 243 | 119 | 119 | 119 | 119 | 0.962 |
| Before | After | p-Value | |
|---|---|---|---|
| Heart rate (beat·min−1) | 147 (8) | 119 (9) | <0.001 |
| MAP (mmHg) | 45 (5) | 66 (1) | <0.001 |
| CO (L·min−1) | 8 (2) | 8.6 (2) | 0.510 |
| SVR (dynes·sec·cm−5) | 410 (11) | 572 (9) | <0.001 |
| PRA (mmHg) | 4 (0.2) | 4.5 (0.1) | <0.001 |
| Pmca (mmHg) | 10.3 (0.3) | 11.9 (0.2) | <0.001 |
| PGVR (mmHg) | 6.3 (0.1) | 7.4 (0.1) | <0.001 |
| RVR (mmHg·min·L−1) | 0.8 (0.2) | 0.9 (0.1) | 0.174 |
| Eh | 0.61 (0.01) | 0.62 (0.01) | 0.826 |
| Vs (mL) | 107 | 257 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalkias, A.; Laou, E.; Papagiannakis, N.; Spyropoulos, V.; Kouskouni, E.; Theodoraki, K.; Xanthos, T. Assessment of Dynamic Changes in Stressed Volume and Venous Return during Hyperdynamic Septic Shock. J. Pers. Med. 2022, 12, 724. https://doi.org/10.3390/jpm12050724
Chalkias A, Laou E, Papagiannakis N, Spyropoulos V, Kouskouni E, Theodoraki K, Xanthos T. Assessment of Dynamic Changes in Stressed Volume and Venous Return during Hyperdynamic Septic Shock. Journal of Personalized Medicine. 2022; 12(5):724. https://doi.org/10.3390/jpm12050724
Chicago/Turabian StyleChalkias, Athanasios, Eleni Laou, Nikolaos Papagiannakis, Vaios Spyropoulos, Evaggelia Kouskouni, Kassiani Theodoraki, and Theodoros Xanthos. 2022. "Assessment of Dynamic Changes in Stressed Volume and Venous Return during Hyperdynamic Septic Shock" Journal of Personalized Medicine 12, no. 5: 724. https://doi.org/10.3390/jpm12050724