A Historical Misconception in Clinical Trials of Drugs for Cancer—Age Grouping
Abstract
:1. Introduction
2. Life Stage, Age, and Cancers
2.1. Age-Specific Cancers
2.2. Age Grouping in Clinical Trials
2.3. The Disagreement between Age and Life Stage
2.4. The Difference in Response to Cancer Drug Treatment between Different Life Stages Is More than the Difference between Arbitrarily Divided Age Groups
2.5. Lack of Study on Drugs of Age Specificity
2.6. Current Considerations for Cancer Treatment of Different Ages
2.7. Hormone Levels and Life Stages
2.8. Immune System and Life Stages
3. Cancer Therapy Design and Life Stages
3.1. Body Growth and the Early Drugs of Chemotherapy
3.2. Life Stages and Targeted Therapy
3.3. Life Stages and Immune Checkpoint Inhibitors
3.4. Life Stages and Molecular Radiotherapy
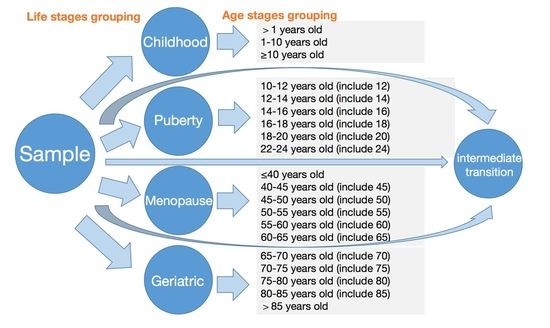
4. Grouping According to Life Stage and Detailed Analyses
5. Conclusions
5.1. Basic Research to Understand Similarities and Differences in Cancer Development and Differences among Life Stages
5.2. Preclinical Test of the Similarities and Differences Using Animal Models
5.3. Standardization of Life Stage Grouping in Drug Test Protocols
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, W. Healthy Long-Lived Human Beings—Working on Life Stages to Break the Limitation of Human Lifespans. Biology 2022, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- Gu, W. It Is Time to Work on the Extension of Body Growth and Reproductive Stages. Rejuvenation Res. 2022, 25, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.P.; Villano, J.L.; McCarthy, B.J. Age-Specific Cancer Incidence Rates Increase Through the Oldest Age Groups. Am. J. Med. Sci. 2014, 348, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, J.-M. Two Hypotheses of Dense Breasts and Viral Infection for explaining incidence rates of breast cancer by age group in Korean women. Epidemiol. Health 2014, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asiri, S.; Asiri, A.; Ulahannan, S.; Alanazi, M.; Humran, A.; Hummadi, A. Incidence Rates of Breast Cancer by Age and Tumor Characteristics Among Saudi Women: Recent Trends. Cureus 2020, 12, e6664. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Society, T.K.B.C.; Oh, M. Effects of interval between age at first pregnancy and age at diagnosis on breast cancer survival according to menopausal status: A register-based study in Korea. BMC Women’s Health 2014, 14, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Siegel, D.; King, J.B. Stage-specific incidence rates and trends of prostate cancer by age, race, and ethnicity, United States, 2004–2014. Ann. Epidemiol. 2018, 28, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Han, L.; Wu, L.; Chen, J.; Sun, H.; Wen, G.; Ji, Y.; Dvorkin, M.; Shi, J.; Pan, Z.; et al. Effect of First-Line Serplulimab vs Placebo Added to Chemotherapy on Survival in Patients With Extensive-Stage Small Cell Lung Cancer. JAMA 2022, 328, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Paz-Ares, L.; Marreaud, S.; Dafni, U.; Oselin, K.; Havel, L.; Esteban, E.; Isla, D.; Martinez-Marti, A.; Faehling, M.; et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB–IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): An interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022, 23, 1274–1286. [Google Scholar] [CrossRef]
- Kogure, Y.; Iwasawa, S.; Saka, H.; Hamamoto, Y.; Kada, A.; Hashimoto, H.; Atagi, S.; Takiguchi, Y.; Ebi, N.; Inoue, A.; et al. Efficacy and safety of carboplatin with nab-paclitaxel versus docetaxel in older patients with squamous non-small-cell lung cancer (CAPITAL): A randomised, multicentre, open-label, phase 3 trial. Lancet Health Longev. 2021, 2, e791–e800. [Google Scholar] [CrossRef]
- Peters, S.; Dziadziuszko, R.; Morabito, A.; Felip, E.; Gadgeel, S.M.; Cheema, P.; Cobo, M.; Andric, Z.; Barrios, C.H.; Yamaguchi, M.; et al. Atezolizumab versus chemotherapy in advanced or metastatic NSCLC with high blood-based tumor mutational burden: Primary analysis of BFAST cohort C randomized phase 3 trial. Nat. Med. 2022, 28, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Westeel, V.; Foucher, P.; Scherpereel, A.; Domas, J.; Girard, P.; Trédaniel, J.; Wislez, M.; Dumont, P.; Quoix, E.; Raffy, O.; et al. Chest CT scan plus x-ray versus chest x-ray for the follow-up of completely resected non-small-cell lung cancer (IFCT-0302): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 1180–1188. [Google Scholar] [CrossRef]
- Lu, S.; Wu, L.; Jian, H.; Chen, Y.; Wang, Q.; Fang, J.; Wang, Z.; Hu, Y.; Sun, M.; Han, L.; et al. Sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): First interim results from a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2022, 23, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, C.; Yao, W.; Wang, Q.; Min, X.; Chen, G.; Xu, X.; Li, X.; Xu, F.; Fang, Y.; et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): A multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, Z.; Sun, Y.; Cao, L.; Ma, Z.; Wu, R.; Yu, Y.; Yao, W.; Chang, J.; Chen, J.; et al. Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): Interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol. 2022, 23, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Chua, B.H.; Link, E.K.; Kunkler, I.H.; Whelan, T.J.; Westenberg, A.H.; Gruber, G.; Bryant, G.; Ahern, V.; Purohit, K.; Graham, P.H.; et al. Radiation doses and fractionation schedules in non-low-risk ductal carcinoma in situ in the breast (BIG 3–07/TROG 07.01): A randomised, factorial, multicentre, open-label, phase 3 study. Lancet 2022, 400, 431–440. [Google Scholar] [CrossRef]
- Wang, B.; Sun, T.; Zhao, Y.; Wang, S.; Zhang, J.; Wang, Z.; Teng, Y.-E.; Cai, L.; Yan, M.; Wang, X.; et al. A randomized phase 3 trial of Gemcitabine or Nab-paclitaxel combined with cisPlatin as first-line treatment in patients with metastatic triple-negative breast cancer. Nat. Commun. 2022, 13, 1–7. [Google Scholar] [CrossRef]
- Tripathy, D.; Tolaney, S.M.; Seidman, A.D.; Anders, C.K.; Ibrahim, N.; Rugo, H.S.; Twelves, C.; Diéras, V.; Müller, V.; Du, Y.; et al. Treatment With Etirinotecan Pegol for Patients With Metastatic Breast Cancer and Brain Metastases. JAMA Oncol. 2022, 8, 1047. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, Q.; Zhang, P.; Hu, X.; Li, W.; Tong, Z.; Sun, T.; Teng, Y.; Wu, X.; Ouyang, Q.; et al. Dalpiciclib or placebo plus fulvestrant in hormone receptor-positive and HER2-negative advanced breast cancer: A randomized, phase 3 trial. Nat. Med. 2021, 27, 1904–1909. [Google Scholar] [CrossRef] [PubMed]
- Del Mastro, L.; Mansutti, M.; Bisagni, G.; Ponzone, R.; Durando, A.; Amaducci, L.; Campadelli, E.; Cognetti, F.; Frassoldati, A.; Michelotti, A.; et al. Extended therapy with letrozole as adjuvant treatment of postmenopausal patients with early-stage breast cancer: A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 1458–1467. [Google Scholar] [CrossRef]
- van der Voort, A.; van Ramshorst, M.S.; van Werkhoven, E.D.; Mandjes, I.A.; Kemper, I.; Vulink, A.J.; Oving, I.M.; Honkoop, A.H.; Tick, L.W.; van de Wouw, A.J.; et al. Three-Year Follow-up of Neoadjuvant Chemotherapy With or Without Anthracyclines in the Presence of Dual ERBB2 Blockade in Patients With ERBB2-Positive Breast Cancer. JAMA Oncol. 2021, 7, 978. [Google Scholar] [CrossRef]
- Mayer, E.L.; Dueck, A.C.; Martin, M.; Rubovszky, G.; Burstein, H.J.; Bellet-Ezquerra, M.; Miller, K.D.; Zdenkowski, N.; Winer, E.P.; Pfeiler, G.; et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): Interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2021, 22, 212–222. [Google Scholar] [CrossRef]
- Yu, K.-D.; Ge, J.-Y.; Liu, X.-Y.; Mo, M.; He, M.; Shao, Z.-M. SPECTRUM Investigators Cyclophosphamide-Free Adjuvant Chemotherapy for Ovarian Protection in Young Women With Breast Cancer: A Randomized Phase 3 Trial. JNCI J. Natl. Cancer Inst. 2021, 113, 1352–1359. [Google Scholar] [CrossRef]
- Yang, W.; Cai, J.; Shen, S.; Gao, J.; Yu, J.; Hu, S.; Jiang, H.; Fang, Y.; Liang, C.; Ju, X.; et al. Pulse therapy with vincristine and dexamethasone for childhood acute lymphoblastic leukaemia (CCCG-ALL-2015): An open-label, multicentre, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2021, 22, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.J.; Devidas, M.; Chen, Z.; Salzer, W.L.; Raetz, E.A.; Rabin, K.R.; Heerema, N.A.; Carroll, A.J.; Gastier-Foster, J.M.; Borowitz, M.J.; et al. Outcomes in adolescent and young adult patients (16 to 30 years) compared to younger patients treated for high-risk B-lymphoblastic leukemia: Report from Children’s Oncology Group Study AALL0232. Leukemia 2021, 36, 648–655. [Google Scholar] [CrossRef]
- Locatelli, F.; Zugmaier, G.; Rizzari, C.; Morris, J.D.; Gruhn, B.; Klingebiel, T.; Parasole, R.; Linderkamp, C.; Flotho, C.; Petit, A.; et al. Effect of blinatumomab vs chemotherapy on event-free survival among children with high-risk first-relapse B-cell acute lymphoblastic leukemia: A randomized clinical trial. JAMA 2021, 325, 843–854. [Google Scholar] [CrossRef]
- Brown, P.A.; Ji, L.; Xu, X.; Devidas, M.; Hogan, L.E.; Borowitz, M.J.; Raetz, E.A.; Zugmaier, G.; Sharon, E.; Bernhardt, M.B.; et al. Effect of postreinduction therapy consolidation with blinatumomab vs chemotherapy on disease-free survival in children, adolescents, and young adults with first relapse of B-cell acute lymphoblastic leukemia: A randomized clinical trial. JAMA 2021, 325, 833–842. [Google Scholar] [CrossRef]
- Peters, C.; Dalle, J.-H.; Locatelli, F.; Poetschger, U.; Sedlacek, P.; Buechner, J.; Shaw, P.J.; Staciuk, R.; Ifversen, M.; Pichler, H.; et al. Total Body Irradiation or Chemotherapy Conditioning in Childhood ALL: A Multinational, Randomized, Noninferiority Phase III Study. J. Clin. Oncol. 2021, 39, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Chen, X.; Cai, J.; Yu, J.; Gao, J.; Hu, S.; Zhai, X.; Liang, C.; Ju, X.; Jiang, H.; et al. Effect of dasatinib vs imatinib in the treatment of pediatric philadelphia chromosome-positive acute lymphoblastic leukemia: A randomized clinical trial. JAMA Oncol. 2020, 6, 358–366. [Google Scholar] [CrossRef] [PubMed]
- E Place, A.; E Stevenson, K.; Vrooman, L.M.; Harris, M.H.; Hunt, S.K.; E O’Brien, J.; Supko, J.G.; Asselin, B.L.; Athale, U.H.; A Clavell, L.; et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli l-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05-001): A randomised, open-label phase 3 trial. Lancet Oncol. 2015, 16, 1677–1690. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, P.J.; Chen, B.E.; Gelmon, K.A.; Whelan, T.J.; Ennis, M.; Lemieux, J.; Ligibel, J.A.; Hershman, D.L.; Mayer, I.A.; Hobday, T.J.; et al. Effect of Metformin vs Placebo on Invasive Disease–Free Survival in Patients With Breast Cancer. JAMA 2022, 327, 1963–1973. [Google Scholar] [CrossRef]
- Calcagno, A.; Trunfio, M.; D’Avolio, A.; Di Perri, G.; Bonora, S. The impact of age on antiretroviral drug pharmacokinetics in the treatment of adults living with HIV. Expert Opin. Drug Metab. Toxicol. 2021, 17, 665–676. [Google Scholar] [CrossRef]
- Joseph, S.; Nicolson, T.J.; Hammons, G.; Word, B.; Green-Knox, B.; Lyn-Cook, B. Expression of drug transporters in human kidney: Impact of sex, age, and ethnicity. Biol. Sex Differ. 2015, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Mora, D.C.; Overvåg, G.; Jong, M.C.; Kristoffersen, A.E.; Stavleu, D.C.; Liu, J.; Stub, T. Complementary and alternative medicine modalities used to treat adverse effects of anti-cancer treatment among children and young adults: A systematic review and meta-analysis of randomized controlled trials. BMC Complement. Med. Ther. 2022, 22, 1–21. [Google Scholar] [CrossRef]
- Gianinazzi, M.E.; Kiserud, C.E.; Ruud, E.; Lie, H.C. Who Knows? Information Received, and Knowledge about, Cancer, Treatment and Late Effects in a National Cohort of Long-Term Childhood, Adolescent and Young Adult Cancer Survivors. Cancers 2022, 14, 1534. [Google Scholar] [CrossRef]
- Puts, M.T.; Strohschein, F.J.; Del Giudice, M.E.; Jin, R.; Loucks, A.; Ayala, A.P.; Alibhai, S.H. Role of the geriatrician, primary care practitioner, nurses, and collaboration with oncologists during cancer treatment delivery for older adults: A narrative review of the literature. J. Geriatr. Oncol. 2018, 9, 398–404. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Liu, Y.; Wang, H.-Y.; Xiang, J. The effects of estrogen on targeted cancer therapy drugs. Pharmacol. Res. 2022, 177. [Google Scholar] [CrossRef]
- Wang, L.; Yin, H.; Gu, W. Testosterone and lifespan in males: At the right time and the right level. Andrologia 2022, 54, e14630. [Google Scholar] [CrossRef] [PubMed]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, L. Evolution of cancer treatment and evolving challenges. Health Manag. Forum 2017, 31, 26–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ram, P.A.; Park, S.-H.; Choi, H.K.; Waxman, D.J. Growth Hormone Activation of Stat 1, Stat 3, and Stat 5 in Rat Liver. J. Biol. Chem. 1996, 271, 5929–5940. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.J.; Elder, M.J.; Yang, C.; Sitnikova, S.I.; Irving, L.; Hansen, A.; Hair, J.; Jones, D.C.; Hasani, S.; Wang, B.; et al. Design and Efficacy of a Monovalent Bispecific PD-1/CTLA4 Antibody That Enhances CTLA4 Blockade on PD-1+ Activated T Cells. Cancer Discov. 2021, 11, 1100–1117. [Google Scholar] [CrossRef] [PubMed]
- Steinfeld, A.D.; Diamond, J.J.; Hanks, G.E.; Coia, L.R.; Kramer, S. Patient Age as a Factor in Radiotherapy. J. Am. Geriatr. Soc. 1989, 37, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Murphy, C.C.; Pruitt, S.L.; Rashdan, S.; Rahimi, A.; Gerber, D.E. Potential Impact of Revised NCI Eligibility Criteria Guidance: Prior Malignancy Exclusion in Breast Cancer Clinical Trials. J. Natl. Compr. Cancer Netw. 2022, 20, 792–799.e4. [Google Scholar] [CrossRef]
- Larkin, H.D. New Cancer Clinical Trial Guidance. JAMA 2022, 327, 1219. [Google Scholar] [CrossRef]


| First Author/Reference | Disease | Age Groups in Treament Population | Age Groups in Ontrol/Other Treatment Population | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | ≥75 | <75 | 65–75 | <65 | ≥65 | Median | ≥75 | <75 | 65–75 | <65 | ≥65 | ||
| Cheng [8] | Extensive-Stage Small Cell Lung Cancer | 63 (28–76) | 235 (60.4) | 62 (31–83) | 119 (60.7) | ||||||||
| O’Brien [9] | completely resected stage IB-IIIA non-small-cell lung cancer | 65 (59.0–70.0) | 285 (48%) | 305 (52%) | 65 (59.0–70.0) | 273 (47%) | 314 (53%) | ||||||
| 64.5 (60.0–69.5) | 84 (50%) | 84 (50%) | 65.0 (58.0–71.0) | 82 (50%) | 82 (50%) | ||||||||
| Kogure [10] | squamous non-small-cell lung cancer | 76 (73–78) | 61 (64%) | 34 (36%) | 77 (73–80) | 65 (67%) | 32 (33%) | ||||||
| Peters [11] | metastatic NSCLC | 66 (39–89) | 108 (46) | 66 (33–86) | 102 (43) | ||||||||
| 65 (39–89) | 72 (50) | 66 (40–86) | 65 (45) | ||||||||||
| Westeel [12] | completely resected non-small-cell lung cancer | 63.0 (56.7–70.5) | |||||||||||
| Lu [13] | EGFR-mutated non-squamous non-small-cell lung cancer | 59 (32–75) | 104 | 44 | 57 (33–78) | 115 | 36 | ||||||
| Wang [14] | extensive-stage small-cell lung cancer | 62 (55–66) | 155 (67%) | 75 (33%) | 62 (56–67) | 147 (63%) | 85 (37%) | ||||||
| Saji [15] | small-sized peripheral non-small-cell lung cancer | 67 (35–85) | 211 | 341 | 67 (32–83) | 211 | 343 | ||||||
| Forde [16] | Resectable Lung Cancer | 64 (41–82) | 93 (52.0) | 86 (48.0) | 65 (34–84) | 83 (46.4) | 96 (53.6) | ||||||
| Zhou [17] | metastatic non-small-cell lung cancer | 62.0 (56.0–67.0) | 202 (63%) | 118 (37%) | 64.0 (56.0–68.0) | 91 (57%) | 68 (43%) | ||||||
| Hellmann [18] | Advanced Non-Small-Cell Lung Cancer | 64 (26–87) | 58 (9.9) | 219 (37.6) | 306 (52.5) | 64 (29–87) | 55 (9.4) | 223 (38.3) | 305 (52.3) | ||||
| First Author/Reference | Disease | Age Groups in Treament Population | Age Groups in Ontrol/Other Treatment Population | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age groups | Median | ≥70 | 60–69 | 50–59 | <50 | Median | ≥70 | 60–69 | 50–59 | <50 | |
| Chua [19] | Non-low-risk ductal carcinoma in situ in the breast | 58 (52–64) | 74 (9%) | 292 (36%) | 306 (38%) | 133 (17%) | 57 (51–65) | 71 (9%) | 267 (33%) | 334 (42%) | 131 (16%) |
| Age groups | Median | ≥40 | <40 | Median | ≥40 | <40 | |||||
| Wang [20] | Metastatic triple-negative breast cancer | 50 (22–69) | 101 (79.5) | 26 (20.5) | 52 (30–75) | 107 (85.0) | 19 (15.0) | ||||
| Age groups | Median | Median | |||||||||
| Tripathy [21] | Metastatic Breast Cancer and Brain Metastases | 53 (27–79) | 52 (24–77) | ||||||||
| Age groups | Median | <65 | ≥65 | Median | <65 | ≥65 | |||||
| Xu [22] | Hormone receptor-positive and HER2-negative advanced breast cancer | 211 | 30 | 108 | 12 | ||||||
| Age groups | Median | ≥76 | 65–75 | 55–64 | <55 | Median | ≥76 | 65–75 | 55–64 | <55 | |
| Del Mastro [23] | Early-stage breast cancer | 60 (54–67) | 58 | 304 | 393 | 275 | 61 (54–68) | 56 | 343 | 386 | 271 |
| Age groups | median (IQR) | ≥50 | <50 | median (IQR) | ≥50 | <50 | |||||
| van der Voort [24] | ERBB2-Positive Breast Cancer | 49 (43–55) | 1101 | 118 | 48(43-56) | 100 | 119 | ||||
| Age groups | median (IQR) | >50 | ≤50 | median (IQR) | >50 | ≤50 | |||||
| Mayer [25] | Early breast cancer | 52 (45–61) | 1573 | 1309 | 52 (45–60) | 1370 | 1304 | ||||
| Age groups | Median age(IQR) | ≤35 | >35 | Median age (IQR) | ≤35 | >35 | |||||
| Yu [26] | Young Women With Breast Cancer | 35 (32–38) | 145 (55.6) | 116 (44.4) | 35 (31-37) | 139 (53.5) | 121 (46.5) | ||||
| First Author/Reference | Disease | Age Groups in Treatment Population | Age Groups in Control/Other Treatment Population | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | ≥10 | 1–10 | <1 | Median | ≥10 | 1–10 | <1 | ||||||
| Yang [27] | Acute lymphoblastic leukemia | 21 (1.46) | 1421 (98.54) | 0 | 23 (1.55) | 1458 (98.45) | 0 | ||||||
| 263 (24.56) | 777 (72.55) | 31 (2.90) | 265 (25.00) | 758 (71.51) | 37 (3.49) | ||||||||
| Age groups | Total | 16–30 | 10–15 | 1–9 | Analysis | ≥16 Years | <16 Years | ||||||
| Burke [28] | High-risk B-lymphoblastic leukemia | 3040 | 20% | 47% | 33% | 597 | 2443 | ||||||
| Age groups | Median | 10–18 | 1–9 | Median | 10–18 | 1–9 | |||||||
| Locatelli [29] | High-risk First-Relapse B-Cell Acute Lymphoblastic Leukemia | 6 (1–17) | 15 (27.8) | 39 (72.2) | 5 (1-17) | 16 (29.6) | 38 (70.4) | ||||||
| Age groups | Median (IQR) | 21–27 | 18–20 | 13–17 | 10–12 | 1–9 | Median (IQR) | 21–27 | 18–20 | 13–17 | 10–12 | 1–9 | |
| Brown [30] | First Relapse of B-Cell Acute Lymphoblastic Leukemia | 9 (6–16) | 7 (6.7) | 8 (7.6) | 25 (23.8) | 10 (9.5) | 55 (52.4) | 9 (5–16) | 8 (7.8) | 10 (9.7) | 19 (18.4) | 11 (10.7) | 55 (53.4) |
| Age groups | Total | >14 | >10–14 | >6–10 | 4–6 | Median | >14 | >10–14 | >6–10 | 4–6 | |||
| Peters [31] | Childhood acute lymphoblastic leukemia | 212 | 48 (23%) | 64 (30%) | 66 (31%) | 34(16%) | 201 | 62 (31%) | 42 (21%) | 75 (37%) | 22 (11%) | ||
| Age groups | ≥10 | 1–9 | ≥10 | 1–9 | |||||||||
| Shen [32] | Pediatric Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia | 60 | 123 | X | X | ||||||||
| Age groups | Overall | ≥10 | <10 | ≥10 | <10 | ||||||||
| Place [33] | Newly diagnosed childhood acute lymphoblastic leukaemia | 551 | 55 (24%) | 176 (76%) | 67 (29%) | 165 (71%) | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Yao, L.; Alamoudi, A.J.; Aleya, L.; Gu, W. A Historical Misconception in Clinical Trials of Drugs for Cancer—Age Grouping. J. Pers. Med. 2022, 12, 1998. https://doi.org/10.3390/jpm12121998
Chen J, Yao L, Alamoudi AJ, Aleya L, Gu W. A Historical Misconception in Clinical Trials of Drugs for Cancer—Age Grouping. Journal of Personalized Medicine. 2022; 12(12):1998. https://doi.org/10.3390/jpm12121998
Chicago/Turabian StyleChen, Jingyu, Lan Yao, Abdulmohsin J. Alamoudi, Lotfi Aleya, and Weikuan Gu. 2022. "A Historical Misconception in Clinical Trials of Drugs for Cancer—Age Grouping" Journal of Personalized Medicine 12, no. 12: 1998. https://doi.org/10.3390/jpm12121998