Minced Cartilage Is a One-Step Cartilage Repair Procedure for Small Defects in the Knee—A Systematic-Review and Meta-Analysis
Abstract
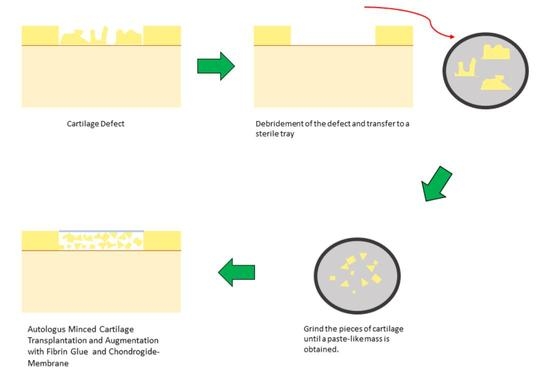
:1. Introduction
2. Methods
2.1. Search Strategy
| # | Query | Results |
| 1 | exp Knee Joint/or exp Cartilage, Articular/or exp Cartilage/or exp Orthopedic Procedures/ | 424,841 |
| 2 | exp Rehabilitation/mt, su, td [Methods, Surgery, Trends] | 70,703 |
| 3 | exp Cartilage/or exp Hyaline Cartilage/or exp Cartilage, Articular/ | 87,137 |
| 4 | exp Treatment Outcome/ | 1,102,955 |
| 5 | 2 or 4 | 1,156,746 |
| 6 | 1 and 5 | 82,188 |
| 7 | 3 and 6 | 6059 |
| 8 | exp Arthroscopy/ae, mt, rh, st, td [Adverse Effects, Methods, Rehabilitation, Standards, Trends] | 10,593 |
| 9 | 7 and 8 | 572 |
| 10 | minced cartilage.mp. | 17 |
| 11 | minced.mp. | 2446 |
| 12 | cartilage.mp. | 98,087 |
| 13 | 11 and 12 | 65 |
| 14 | 10 or 13 | 65 |
| 15 | 9 or 14 | 637 |
2.2. Eligibility
2.3. Primary Outcome Criteria
2.4. Statistics
3. Results
3.1. Study Selection
3.2. Risk of Bias Assessment
3.3. Clinical Outcome
3.3.1. Clinical Scores at 12-Month Post-Surgery
3.3.2. Clinical Scores at 24-Month Post-Surgery
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hjelle, K.; Solheim, E.; Strand, T.; Muri, R.; Brittberg, M. Articular cartilage defects in 1,000 knee arthroscopies. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2002, 18, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Strauss, E.J.; Fonseca, L.E.; Shah, M.R.; Yorum, T. Management of focal cartilage defects in the knee—Is ACI the answer? Bull. NYU Hosp. Jt. Dis. 2011, 69, 63–72. [Google Scholar] [PubMed]
- Chimutengwende-Gordon, M.; Donaldson, J.; Bentley, G. Current solutions for the treatment of chronic articular cartilage defects in the knee. EFORT Open Rev. 2020, 5, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, G.M.; Calek, A.-K.; Preiss, S. Second-Generation Autologous Minced Cartilage Repair Technique. Arthrosc. Tech. 2017, 6, e127–e131. [Google Scholar] [CrossRef] [Green Version]
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic Science of Articular Cartilage. Clin. Sports Med. 2017, 36, 413–425. [Google Scholar] [CrossRef]
- Ulrich-Vinther, M.; Maloney, M.D.; Schwarz, E.M.; Rosier, R.; O’Keefe, R.J. Articular cartilage biology. J. Am. Acad. Orthop. Surg. 2003, 11, 421–430. [Google Scholar] [CrossRef]
- Borrelli, J.; Olson, S.A.; Godbout, C.; Schemitsch, E.H.; Stannard, J.P.; Giannoudis, P.V. Understanding Articular Cartilage Injury and Potential Treatments. J. Orthop. Trauma. 2019, 33 (Suppl. S6), S6–S12. [Google Scholar] [CrossRef]
- Simon, T.M.; Jackson, D.W. Articular Cartilage: Injury Pathways and Treatment Options. Sports Med. Arthrosc. Rev. 2018, 26, 31–39. [Google Scholar] [CrossRef]
- Tsuyuguchi, Y.; Nakasa, T.; Ishikawa, M.; Miyaki, S.; Matsushita, R.; Kanemitsu, M.; Adachi, N. The Benefit of Minced Cartilage Over Isolated Chondrocytes in Atelocollagen Gel on Chondrocyte Proliferation and Migration. Cartilage 2021, 12, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Evuarherhe, A., Jr.; Condron, N.B.; Knapik, D.M.; Haunschild, E.D.; Gilat, R.; Huddleston, H.P.; Kaiser, J.T.; Parvaresh, K.C.; Wagner, K.R.; Chubinskaya, S.; et al. Effect of Mechanical Mincing on Minimally Manipulated Articular Cartilage for Surgical Transplantation. Am. J. Sports Med. 2022, 50, 2515–2525, Erratum in Am. J. Sports Med. 2022, 26, 3635465221131042. [Google Scholar] [CrossRef]
- Salzmann, G.M.; Ossendorff, R.; Gilat, R.; Cole, B.J. Autologous Minced Cartilage Implantation for Treatment of Chondral and Osteochondral Lesions in the Knee Joint: An Overview. Cartilage 2020, 13, 1947603520942952. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 21, 339. Available online: https://www.bmj.com/content/339/bmj.b2700 (accessed on 15 July 2022). [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 12, 355. Available online: https://www.bmj.com/content/355/bmj.i4919 (accessed on 15 July 2022). [CrossRef] [PubMed] [Green Version]
- Coleman, B.D.; Khan, K.M.; Maffulli, N.; Cook, J.L.; Wark, J.D. Studies of surgical outcome after patellar tendinopathy: Clinical significance of methodological deficiencies and guidelines for future studies. Vic. Inst. Sport Tendon. Study Group. Scand. J. Med. Sci. Sports. 2000, 10, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Hsu, P.; Zhu, Z.; Zhang, C. Current surgical options and innovation for repairing articular cartilage defects in the femoral head. J. Orthop. Transl. 2020, 21, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurst, J.M.; Steadman, J.R.; O’Brien, L.; Rodkey, W.G.; Briggs, K.K. Rehabilitation following microfracture for chondral injury in the knee. Clin. Sports Med. 2010, 29, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Steadman, J.R.; Miller, B.S.; Karas, S.G.; Schlegel, T.F.; Briggs, K.K.; Hawkins, R.J. The microfracture technique in the treatment of full-thickness chondral lesions of the knee in National Football League players. J. Knee Surg. 2003, 16, 83–86. [Google Scholar]
- Pollard, T.D.; Earnshaw, W.C.; Lippincott-Schwartz, J.; Johnson, G.T. (Eds.) Chapter 32—Connective Tissues. In Cell Biology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 555–570. Available online: https://www.sciencedirect.com/science/article/pii/B9780323341264000323 (accessed on 15 July 2022).
- Gobbi, A.; Karnatzikos, G.; Kumar, A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2014, 22, 1986–1996. [Google Scholar] [CrossRef]
- Orth, P.; Gao, L.; Madry, H. Microfracture for cartilage repair in the knee: A systematic review of the contemporary literature. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 670–706. [Google Scholar] [CrossRef] [PubMed]
- Steinwachs, M.R.; Gille, J.; Volz, M.; Anders, S.; Jakob, R.; De Girolamo, L.; Volpi, P.; Schiavone-Panni, A.; Scheffler, S.; Reiss, E.; et al. Systematic Review and Meta-Analysis of the Clinical Evidence on the Use of Autologous Matrix-Induced Chondrogenesis in the Knee. Cartilage 2021, 13, 42S–56S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migliorini, F.; Eschweiler, J.; Schenker, H.; Baroncini, A.; Tingart, M.; Maffulli, N. Surgical management of focal chondral defects of the knee: A Bayesian network meta-analysis. J. Orthop. Surg. 2021, 16, 543. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, N.; Jakob, R.P.; Pagenstert, G.; Tannast, M.; Petek, D. Stable clinical long term results after AMIC in the aligned knee. Arch. Orthop. Trauma Surg. 2021, 141, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Iizawa, N.; Takai, S.; Majima, T. Maturation process of regenerated tissues after single-stage simultaneous autologous particulated cartilage implantation and open wedge high tibial osteotomy for articular cartilage defects with medial osteoarthritis of bilateral knees: A case report. BMC Musculoskelet. Disord. 2021, 22, 502. [Google Scholar] [CrossRef] [PubMed]
- Wasiak, J.; Clar, C.; Villanueva, E. Autologous cartilage implantation for full thickness articular cartilage defects of the knee. Cochrane Database Syst. Rev. 2006, 3, CD003323. [Google Scholar]
- Gooding, C.R.; Bartlett, W.; Bentley, G.; Skinner, J.A.; Carrington, R.; Flanagan, A. A prospective, ranomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: Periosteum covered versus type I/III collagen covered. Knee 2006, 13, 203–210. [Google Scholar] [CrossRef]
- Mistry, H.; Connock, M.; Pink, J.; Shyangdan, D.; Clar, C.; Royle, P.; Court, R.; Biant, L.C.; Metcalfe, A.; Waugh, N. Autologous chondrocyte implantation in the knee: Systematic review and economic evaluation. Health Technol. Assess. Winch. Engl. 2017, 21, 1–294. [Google Scholar] [CrossRef] [Green Version]
- Zeifang, F.; Oberle, D.; Nierhoff, C.; Richter, W.; Moradi, B.; Schmitt, H. Autologous Chondrocyte Implantation Using the Original Periosteum-Cover Technique versus Matrix-Associated Autologous Chondrocyte Implantation: A Randomized Clinical Trial. Am. J. Sports Med. 2010, 38, 924–933. [Google Scholar] [CrossRef]
- Migliorini, F.; Eschweiler, J.; Spiezia, F.; van de Wall, B.J.M.; Knobe, M.; Tingart, M.; Maffulli, N. Arthroscopy versus mini-arthrotomy approach for matrix-induced autologous chondrocyte implantation in the knee: A systematic review. J. Orthop. Traumatol. Off. J. Ital. Soc. Orthop. Traumatol. 2021, 22, 23. [Google Scholar] [CrossRef]
- Jones, K.J.; Cash, B.M. Matrix-Induced Autologous Chondrocyte Implantation With Autologous Bone Grafting for Osteochondral Lesions of the Femoral Trochlea. Arthrosc. Tech. 2019, 8, e259–e266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barié, A.; Kruck, P.; Sorbi, R.; Rehnitz, C.; Oberle, D.; Walker, T.; Zeifang, F.; Moradi, B. Prospective Long-term Follow-up of Autologous Chondrocyte Implantation With Periosteum Versus Matrix-Associated Autologous Chondrocyte Implantation: A Randomized Clinical Trial. Am. J. Sports Med. 2020, 48, 2230–2241. [Google Scholar] [CrossRef] [PubMed]
- Valtanen, R.S.; Arshi, A.; Kelley, B.V.; Fabricant, P.D.; Jones, K.J. Articular Cartilage Repair of the Pediatric and Adolescent Knee with Regard to Minimal Clinically Important Difference: A Systematic Review. Cartilage 2020, 11, 9–18. [Google Scholar] [CrossRef]
- Kim, J.-H.; Heo, J.-W.; Lee, D.-H. Clinical and Radiological Outcomes After Autologous Matrix-Induced Chondrogenesis Versus Microfracture of the Knee: A Systematic Review and Meta-analysis With a Minimum 2-Year Follow-up. Orthop. J. Sports Med. 2020, 8, 2325967120959280. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, G.M.; Buchberger, M.S.; Stoddart, M.J.; Grad, S.; Milz, S.; Niemyer, P.; Sudkamp, N.P.; Imhoff, A.B.; Alini, M. Varying Regional Topology Within Knee Articular Chondrocytes Under Simulated In Vivo Conditions. Tissue Eng. Part A 2011, 17, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Aurich, M.; Hofmann, G.O.; Best, N.; Rolauffs, B. Induced Redifferentiation of Human Chondrocytes from Articular Cartilage Lesion in Alginate Bead Culture After Monolayer Dedifferentiation: An Alternative Cell Source for Cell-Based Therapies? Tissue Eng. Part A 2018, 24, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Aurich, M.; Hofmann, G.O.; Rolauffs, B. Tissue engineering-relevant characteristics of ex vivo and monolayer-expanded chondrocytes from the notch versus trochlea of human knee joints. Int. Orthop. 2017, 41, 2327–2335. [Google Scholar] [CrossRef]
- Lauer, J.C.; Selig, M.; Hart, M.L.; Kurz, B.; Rolauffs, B. Articular Chondrocyte Phenotype Regulation through the Cytoskeleton and the Signaling Processes That Originate from or Converge on the Cytoskeleton: Towards a Novel Understanding of the Intersection between Actin Dynamics and Chondrogenic Function. Int. J. Mol. Sci. 2021, 22, 3279. [Google Scholar] [CrossRef]
- Albrecht, F.H. Closure of joint cartilage defects using cartilage fragments and fibrin glue. Fortschr. Med. 1983, 101, 1650–1652. [Google Scholar]
- Christensen, B.B.; Olesen, M.L.; Hede, K.T.C.; Bergholt, N.L.; Foldager, C.B.; Lind, M. Particulated Cartilage for Chondral and Osteochondral Repair: A Review. Cartilage 2021, 13, 1047S–1057S. [Google Scholar] [CrossRef]
- Christensen, B.B.; Foldager, C.B.; Jensen, J.; Lind, M. Autologous Dual-Tissue Transplantation for Osteochondral Repair: Early Clinical and Radiological Results. Cartilage 2015, 6, 166–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farr, J.; Tabet, S.K.; Margerrison, E.; Cole, B.J. Clinical, Radiographic, and Histological Outcomes After Cartilage Repair With Particulated Juvenile Articular Cartilage: A 2-Year Prospective Study. Am. J. Sports Med. 2014, 42, 1417–1425. [Google Scholar] [CrossRef] [PubMed]





| Risk of Bias Preintervention and at-Intervaention Domains | Risk of Bias Post-Intervention Domains | |||||||
|---|---|---|---|---|---|---|---|---|
| study | Bias due to confounding | Bias due to selection of participants into study | Bias in classification of intervention | Bias due to deviation from intended intervention | Bias due to missing data | Bias in measurement of outcome | Bias in the selection of reported outcome | Overall assessment of bias |
| Christensen | Low | Low | Moderate | Low | moderate | moderate | Low | moderate |
| Farr | Low | Low | Low | low | moderate | moderate | low | moderate |
| Cole | low | low | low | low | low | low | low | low |
| Buckwalter | serious | moderate | Low | low | moderate | moderate | low | serious |
| Massen | serious | serious | low | Low | low | moderate | low | serious |
 .
.| Modified Coleman Methology Score | |||||
|---|---|---|---|---|---|
| Christensen et al. | Cole et al. | Farr et al. | Buckwalter et al. | Massen et al. | |
| PART A | |||||
| Study size | 0 | 0 | 0 | 0 | 0 |
| Mean follow up | 0 | 0 | 0 | 0 | 0 |
| Percent of Patients with follow up | 3 | 5 | 5 | 0 | 5 |
| Numbers of interventions per group | 10 | 10 | 10 | 10 | 10 |
| Type of study | 10 | 15 | 10 | 10 | 0 |
| Diagnostic certainly | 5 | 5 | 5 | 5 | 5 |
| Description of surgical technique | 5 | 5 | 5 | 5 | 5 |
| Definition of postoperativ rehabilitation | 5 | 5 | 5 | 5 | 0 |
| PART B | |||||
| Outcome criteria | 10 | 10 | 10 | 8 | 7 |
| Procedure of assaying outcomes | 11 | 11 | 11 | 11 | 11 |
| Description of subject selection Prozess | 5 | 10 | 5 | 5 | 5 |
| 64 | 76 | 66 | 59 | 48 |
| Study | Number of Patients | Age (y) | Male-Female | Defect Location | Defect Size (cm2) | Defect Type | Operation Method |
|---|---|---|---|---|---|---|---|
| Christensen | 8 Patients | 32 +/− 7 | female 3 male 5 | Femur condyle 7 Trochlea 1 | 3.1 (1.5-4.7) | Osteochondritis dissecans | Minced cartilage with fibrin glue, additional bine reconstrution |
| Cole | 20 Patients 24 Lesions | 32.7 +/− 8.8 | female 6 male 14 | Femur condyle 14 Trochlea 10 | 2.75 +/− 1.5 | ICRS grade III 20 ICRS grade IV 4 | Minced cartilage with fibrin glue and CAIS Scaffold |
| Farr | 25 Patients 29 Lesions | 37 +/− 11.1 | femlae 7 male 18 | Femur condyle 18 Trochlea 11 | 2.7 +/− 0.8 | ICRS grade III 23 ICRS grade IV 6 | Juvenile minced cartilage allograft fixed with fibrin glue |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frodl, A.; Siegel, M.; Fuchs, A.; Wagner, F.C.; Schmal, H.; Izadpanah, K.; Yilmaz, T. Minced Cartilage Is a One-Step Cartilage Repair Procedure for Small Defects in the Knee—A Systematic-Review and Meta-Analysis. J. Pers. Med. 2022, 12, 1923. https://doi.org/10.3390/jpm12111923
Frodl A, Siegel M, Fuchs A, Wagner FC, Schmal H, Izadpanah K, Yilmaz T. Minced Cartilage Is a One-Step Cartilage Repair Procedure for Small Defects in the Knee—A Systematic-Review and Meta-Analysis. Journal of Personalized Medicine. 2022; 12(11):1923. https://doi.org/10.3390/jpm12111923
Chicago/Turabian StyleFrodl, Andreas, Markus Siegel, Andreas Fuchs, Ferdinand C. Wagner, Hagen Schmal, Kaywan Izadpanah, and Tayfun Yilmaz. 2022. "Minced Cartilage Is a One-Step Cartilage Repair Procedure for Small Defects in the Knee—A Systematic-Review and Meta-Analysis" Journal of Personalized Medicine 12, no. 11: 1923. https://doi.org/10.3390/jpm12111923