Prolonged Maternal Separation Reduces Anxiety State and Increases Compulsive Burying Activity in the Offspring of BALB/c Mice
Abstract
:1. Introduction
2. Methods
2.1. Animals Care and Husbandry
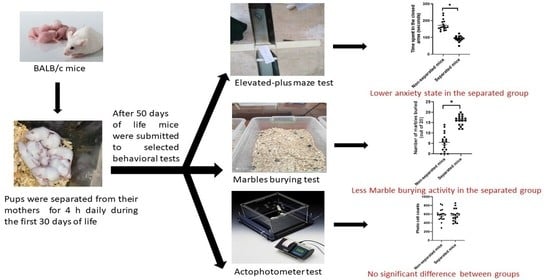
2.2. Maternal Separation Protocol
2.3. Elevated plus Maze Test
2.4. Marble Burying Test
2.5. Measurement of Locomotor Activity
2.6. Statistical Analysis
3. Results
3.1. Elevated plus Maze Test
3.2. Marble Burying Test
3.3. Measurement of Locomotor Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nakamura, J.S.; Kim, E.S.; Rentscher, K.E.; Bower, J.E.; Kuhlman, K.R. Early-life stress, depressive symptoms, and inflammation: The role of social factors. Aging Ment. Health 2022, 26, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Offerman, E.C.P.; Asselman, M.W.; Bolling, F.; Helmond, P.; Stams, G.-J.J.M.; Lindauer, R.J.L. Prevalence of adverse childhood experiences in students with emotional and behavioral disorders in special education schools from a multi-informant perspective. Int. J. Environ. Res. Public Health 2022, 19, 3411. [Google Scholar] [CrossRef] [PubMed]
- Sharratt, K.; Mason, S.J.; Kirkman, G.; Willmott, D.; McDermott, D.; Timmins, S.; Wager, N.M. Childhood abuse and neglect, exposure to domestic violence and sibling violence: Profiles and associations with sociodemographic variables and mental health indicators. J. Interpers. Violence 2022. [Google Scholar] [CrossRef] [PubMed]
- Moody, G.; Cannings-John, R.; Hood, K.; Kemp, A.; Robling, M. Establishing the international prevalence of self-reported child maltreatment: A systematic review by maltreatment type and gender. BMC Public Health 2018, 18, 1164. [Google Scholar] [CrossRef] [Green Version]
- Pace, C.S.; Muzi, S.; Rogier, G.; Meinero, L.L.; Marcenaro, S. The Adverse Childhood Experiences–International Questionnaire (ACE-IQ) in community samples around the world: A systematic review (part I). Child Abuse Negl. 2022, 129, 105640. [Google Scholar] [CrossRef]
- Lochner, C.; du Toit, P.L.; Zungu-Dirwayi, N.; Marais, A.; van Kradenburg, J.; Seedat, S.; Niehaus, D.J.H.; Stein, D.J. Childhood trauma in obsessive-compulsive disorder, trichotillomania, and controls. Depress. Anxiety 2002, 15, 66–68. [Google Scholar]
- Oginga, F.O.; Magwai, T.; Shangase, K.B.; Xulu, K.R.; Mpofana, T. Early Life Stress and Brain Plasticity: From Alterations of Brain Morphology to Development of Psychopathology. NeuroSci 2022, 3, 104–110. [Google Scholar] [CrossRef]
- Zovetti, N.; Perlini, C.; Brambilla, P.; Bellani, M. Childhood adversities and bipolar disorder: A neuroimaging focus. Epidemiol. Psychiatr. Sci. 2022, 31, e12. [Google Scholar] [CrossRef]
- Rachel, C.; Roman, N.V.; Donga, G.T. The Contribution of Parental Factors to Adolescents’ Deviant Behaviour in South Africa: Evidence from Three Rural Communities in South Africa. Soc. Sci. 2022, 11, 152. [Google Scholar] [CrossRef]
- Nilaweera, D.; Freak-Poli, R.; Gurvich, C.; Ritchie, K.; Chaudieu, I.; Ancelin, M.-L.; Ryan, J. The association between adverse childhood events and later-life cognitive function and dementia risk. J. Affect. Disord. 2022, 304, 128–132. [Google Scholar] [CrossRef]
- Marusak, H.A.; Kuruvadi, N.; Vila, A.M.; Shattuck, D.W.; Joshi, S.H.; Joshi, A.A.; Jella, P.K.; Thomason, M.E. Interactive effects of BDNF Val66Met genotype and trauma on limbic brain anatomy in childhood. Eur. Child. Adolesc. Psychiatry 2016, 25, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Calem, M.; Bromis, K.; McGuire, P.; Morgan, C.; Kempton, M.J. Meta-analysis of associations between childhood adversity and hippocampus and amygdala volume in non-clinical and general population samples. Neuroimage Clin. 2017, 14, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.J.; Dalvie, S.; Cuzen, N.L.; Cardenas, V.; Fein, G.; Stein, D.J. Childhood adversity is linked to differential brain volumes in adolescents with alcohol use disorder: A voxel-based morphometry study. Metab. Brain Dis. 2014, 29, 311–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasecka, A.; Lutz, P.-E.; Tanti, A.; Mechawar, N.; Turecki, G.; Côté, D.C. Early Life Adversity Leads to Demyelination in the Anterior Cingulate Cortex. In Proceedings of the Novel Techniques in Microscopy; Optical Society of America, Tucson, AZ, USA, 14–17April 2019; p. JT4A-15. [Google Scholar]
- Park, A.T.; Leonard, J.A.; Saxler, P.K.; Cyr, A.B.; Gabrieli, J.D.E.; Mackey, A.P. Amygdala–medial prefrontal cortex connectivity relates to stress and mental health in early childhood. Soc. Cogn. Affect. Neurosci. 2018, 13, 430–439. [Google Scholar] [CrossRef] [Green Version]
- Herringa, R.J.; Burghy, C.A.; Stodola, D.E.; Fox, M.E.; Davidson, R.J.; Essex, M.J. Enhanced prefrontal-amygdala connectivity following childhood adversity as a protective mechanism against internalizing in adolescence. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 326–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontana, B.D.; Cleal, M.; Norton, W.H.J.; Parker, M.O. The impact of chronic unpredictable early-life stress (CUELS) on boldness and stress-reactivity: Differential effects of stress duration and context of testing. Physiol. Behav. 2021, 240, 113526. [Google Scholar] [CrossRef]
- Qin, X.; He, Y.; Wang, N.; Zou, J.-X.; Zhang, Y.-M.; Cao, J.-L.; Pan, B.-X.; Zhang, W.-H. Moderate maternal separation mitigates the altered synaptic transmission and neuronal activation in amygdala by chronic stress in adult mice. Mol. Brain 2019, 12, 111. [Google Scholar] [CrossRef] [Green Version]
- Callaghan, B.L.; Sullivan, R.M.; Howell, B.; Tottenham, N. The international society for developmental psychobiology Sackler symposium: Early adversity and the maturation of emotion circuits—A cross-species analysis. Dev. Psychobiol. 2014, 56, 1635–1650. [Google Scholar] [CrossRef] [Green Version]
- Gee, D.G.; Gabard-Durnam, L.J.; Flannery, J.; Goff, B.; Humphreys, K.L.; Telzer, E.H.; Hare, T.A.; Bookheimer, S.Y.; Tottenham, N. Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. USA 2013, 110, 15638–15643. [Google Scholar] [CrossRef] [Green Version]
- Lovallo, W.R. Early life adversity reduces stress reactivity and enhances impulsive behavior: Implications for health behaviors. Int. J. Psychophysiol. 2013, 90, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Mlouki, I.; Bouanene, I.; Sioud, I.; Bchir, A.; al’Absi, M.; El Mhamdi, S. Impulsivity mediates the impact of early life adversity on high risk behaviors among Tunisian adolescents. Prev. Med. Rep. 2021, 23, 101424. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S. Molecular genetics of obsessive–compulsive disorder: A comprehensive meta-analysis of genetic association studies. Mol. Psychiatry 2013, 18, 799–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stella, V.J. Prodrugs as therapeutics. Expert Opin. Ther. Pat. 2004, 14, 277–280. [Google Scholar] [CrossRef]
- Boger, S.; Ehring, T.; Berberich, G.; Werner, G.G. Impact of childhood maltreatment on obsessive-compulsive disorder symptom severity and treatment outcome. Eur. J. Psychotraumatol. 2020, 11, 1753942. [Google Scholar] [CrossRef]
- Ou, W.; Li, Z.; Zheng, Q.; Chen, W.; Liu, J.; Liu, B.; Zhang, Y. Association between childhood maltreatment and symptoms of obsessive-compulsive disorder: A meta-analysis. Front. Psychiatry 2021, 11, 612586. [Google Scholar] [CrossRef]
- Destree, L.; Brierley, M.-E.E.; Albertella, L.; Jobson, L.; Fontenelle, L.F. The effect of childhood trauma on the severity of obsessive-compulsive symptoms: A systematic review. J. Psychiatr. Res. 2021, 142, 345–360. [Google Scholar] [CrossRef]
- Adams, T.G.; Kelmendi, B.; Brake, C.A.; Gruner, P.; Badour, C.L.; Pittenger, C. The role of stress in the pathogenesis and maintenance of obsessive-compulsive disorder. Chronic Stress 2018, 2. [Google Scholar] [CrossRef] [Green Version]
- NICHD Early Child Care Research Network. The effects of infant child care on infant-mother attachment security: Results of the NICHD study of early child care. Child Dev. 1997, 68, 860–879. [Google Scholar]
- Bowlby, J. Forty-four juvenile thieves: Their characters and home-life (II). Int. J. Psychoanal. 1944, 25, 107–128. [Google Scholar]
- Bowlby, J. Maternal Care and Mental Health; World Health Organization: Geneva, Switzerland, 1951; Volume 2. [Google Scholar]
- McCall, R.B.; Groark, C.J.; Hawk, B.N.; Julian, M.M.; Merz, E.C.; Rosas, J.M.; Muhamedrahimov, R.J.; Palmov, O.I.; Nikiforova, N.V. Early caregiver–child interaction and children’s development: Lessons from the St. Petersburg-USA Orphanage intervention research project. Clin. Child Fam. Psychol. Rev. 2019, 22, 208–224. [Google Scholar] [CrossRef]
- Cramm, H.; McColl, M.A.; Aiken, A.B.; Williams, A. The mental health of military-connected children: A scoping review. J. Child Fam. Stud. 2019, 28, 1725–1735. [Google Scholar] [CrossRef] [Green Version]
- Anglin, D.M.; Cohen, P.R.; Chen, H. Duration of early maternal separation and prediction of schizotypal symptoms from early adolescence to midlife. Schizophr. Res. 2008, 103, 143–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csóka, S.; Simor, P.; Szabó, G.; Kopp, M.S.; Bódizs, R. Early maternal separation, nightmares, and bad dreams: Results from the Hungarostudy Epidemiological Panel. Attach. Hum. Dev. 2011, 13, 125–140. [Google Scholar] [CrossRef]
- Enoch, M.-A. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology 2011, 214, 17–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledo-Rodriguez, M.; Sandi, C. Stress before puberty exerts a sex-and age-related impact on auditory and contextual fear conditioning in the rat. Neural Plast. 2007, 2007, 071203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, J.; Pryce, C.R.; Bettschen, D.; Feldon, J. The maternal separation paradigm and adult emotionality and cognition in male and female Wistar rats. Pharmacol. Biochem. Behav. 1999, 64, 705–715. [Google Scholar] [CrossRef]
- Arborelius, L.; Eklund, M.B. Both long and brief maternal separation produces persistent changes in tissue levels of brain monoamines in middle-aged female rats. Neuroscience 2007, 145, 738–750. [Google Scholar] [CrossRef]
- Sousa, V.C.; Vital, J.; Costenla, A.R.; Batalha, V.L.; Sebastião, A.M.; Ribeiro, J.A.; Lopes, L. V Maternal separation impairs long term-potentiation in CA1-CA3 synapses and hippocampal-dependent memory in old rats. Neurobiol. Aging 2014, 35, 1680–1685. [Google Scholar] [CrossRef]
- Tractenberg, S.G.; Levandowski, M.L.; de Azeredo, L.A.; Orso, R.; Roithmann, L.G.; Hoffmann, E.S.; Brenhouse, H.; Grassi-Oliveira, R. An overview of maternal separation effects on behavioural outcomes in mice: Evidence from a four-stage methodological systematic review. Neurosci. Biobehav. Rev. 2016, 68, 489–503. [Google Scholar] [CrossRef]
- Romeo, R.D.; Mueller, A.; Sisti, H.M.; Ogawa, S.; McEwen, B.S.; Brake, W.G. Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm. Behav. 2003, 43, 561–567. [Google Scholar] [CrossRef]
- Miragaia, A.S.; de Oliveira Wertheimer, G.S.; Consoli, A.C.; Cabbia, R.; Longo, B.M.; Girardi, C.E.N.; Suchecki, D. Maternal deprivation increases anxiety-and depressive-like behaviors in an age-dependent fashion and reduces neuropeptide Y expression in the amygdala and hippocampus of male and female young adult rats. Front. Behav. Neurosci. 2018, 12, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millstein, R.A.; Holmes, A. Effects of repeated maternal separation on anxiety-and depression-related phenotypes in different mouse strains. Neurosci. Biobehav. Rev. 2007, 31, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; San Ho, H.; Song, A.Y.; Low, J.; Je, H.S. Maternal separation does not produce a significant behavioral change in mice. Exp. Neurobiol. 2017, 26, 390. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, L.; Yang, S.; Qin, D.; Wang, J.; Li, C.; Lv, L.; Ma, Y.; Hu, X. Maternal separation produces lasting changes in cortisol and behavior in rhesus monkeys. Proc. Natl. Acad. Sci. USA 2011, 108, 14312–14317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uysal, N.; Şişman, A.R.; Gönenç, S.; Acikgöz, O.; Kayatekin, B.M.; Yalaz, G. Effects Of Repeated Maternal Separation On Oxidative Stress In Adolescent Male and Female Rat Brains. J. Neurol. Sci. 2008, 25, 50–157. [Google Scholar]
- Sullivan, R.M.; Opendak, M. Neurobiology of infant fear and anxiety: Impacts of delayed amygdala development and attachment figure quality. Biol. Psychiatry 2021, 89, 641–650. [Google Scholar] [CrossRef]
- Hui, J.; Zhang, Z.; Liu, S.; Xi, G.; Zhang, X.; Teng, G.-J.; Chan, K.C.; Wu, E.X.; Nie, B.; Shan, B. Hippocampal neurochemistry is involved in the behavioural effects of neonatal maternal separation and their reversal by post-weaning environmental enrichment: A magnetic resonance study. Behav. Brain Res. 2011, 217, 122–127. [Google Scholar] [CrossRef]
- Grochecki, P.; Smaga, I.; Surowka, P.; Marszalek-Grabska, M.; Kalaba, P.; Dragacevic, V.; Kotlinska, P.; Filip, M.; Lubec, G.; Kotlinska, J.H. Novel Dopamine Transporter Inhibitor, CE-123, Ameliorates Spatial Memory Deficits Induced by Maternal Separation in Adolescent Rats: Impact of Sex. Int. J. Mol. Sci. 2022, 23, 10718. [Google Scholar] [CrossRef]
- Colorado, R.A.; Shumake, J.; Conejo, N.M.; Gonzalez-Pardo, H.; Gonzalez-Lima, F. Effects of maternal separation, early handling, and standard facility rearing on orienting and impulsive behavior of adolescent rats. Behav. Process. 2006, 71, 51–58. [Google Scholar] [CrossRef]
- Delavari, F.; Sheibani, V.; Esmaeili-Mahani, S.; Nakhaee, N. Maternal separation and the risk of drug abuse in later life. Addict. Health 2016, 8, 107. [Google Scholar]
- Pellow, S.; File, S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986, 24, 525–529. [Google Scholar] [CrossRef]
- Handley, S.L. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol. Biochem. Behav. 1991, 38, 63–67. [Google Scholar]
- Angoa-Pérez, M.; Kane, M.J.; Briggs, D.I.; Francescutti, D.M.; Kuhn, D.M. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. JoVE J. Vis. Exp. 2013, 82, e50978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, A.; Burant, A.; Bui, N.; Graham, D.; Yuva-Paylor, L.A.; Paylor, R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology 2009, 204, 361–373. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.Y.; Han, S.H.; Woo, R.-S.; Jang, S.H.; Min, S.S. Adolescent mice show anxiety-and aggressive-like behavior and the reduction of long-term potentiation in mossy fiber-CA3 synapses after neonatal maternal separation. Neuroscience 2016, 316, 221–231. [Google Scholar] [CrossRef]
- Mahmoodkhani, M.; Ghasemi, M.; Derafshpour, L.; Amini, M.; Mehranfard, N. Long-term decreases in the expression of calcineurin and GABAa receptors induced by early maternal separation are associated with increased anxiety-like behavior in adult male rats. Dev. Neurosci. 2020, 42, 135–144. [Google Scholar] [CrossRef]
- Sterley, T.-L.; Howells, F.M.; Russell, V.A. Effects of early life trauma are dependent on genetic predisposition: A rat study. Behav. Brain Funct. 2011, 7, 11. [Google Scholar] [CrossRef]
- Bian, Y.; Ma, Y.; Ma, Q.; Yang, L.; Zhu, Q.; Li, W.; Meng, L. Prolonged Maternal Separation Induces the Depression-like Behavior Susceptibility to Chronic Unpredictable Mild Stress Exposure in Mice. Biomed Res. Int. 2021, 2021, 6681397. [Google Scholar] [CrossRef]
- Lundberg, S.; Abelson, K.S.P.; Nylander, I.; Roman, E. Few long-term consequences after prolonged maternal separation in female Wistar rats. PLoS ONE 2017, 12, e0190042. [Google Scholar] [CrossRef] [Green Version]
- Cruz, F.C.; Quadros, I.M.; da Planeta, C.S.; Miczek, K.A. Maternal separation stress in male mice: Long-term increases in alcohol intake. Psychopharmacology 2008, 201, 459–468. [Google Scholar] [CrossRef] [Green Version]
- Jarrar, B.; Al-Doaiss, A.; Shati, A.; Al-Kahtani, M.; Jarrar, Q. Behavioural alterations induced by chronic exposure to 10 nm silicon dioxide nanoparticles. IET Nanobiotechnology 2021, 15, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Tirumalasetti, J.; Patel, M.; Shaikh, U.; Harini, K.; Shankar, J. Evaluation of skeletal muscle relaxant activity of aqueous extract of Nerium oleander flowers in Albino rats. Indian J. Pharmacol. 2015, 47, 409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, S.; Muench, J. The epigenetic impact of adverse childhood experiences through the lens of personalized medicine. Epigenomics 2022, 14, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Lundgaard Donovan, L.; Henningsen, K.; Flou Kristensen, A.; Wiborg, O.; Nieland, J.D.; Lichota, J. Maternal separation followed by chronic mild stress in adulthood is associated with concerted epigenetic regulation of AP-1 complex genes. J. Pers. Med. 2021, 11, 209. [Google Scholar] [CrossRef]
- Krause, B.J.; Artigas, R.; Sciolla, A.F.; Hamilton, J. Epigenetic mechanisms activated by childhood adversity. Epigenomics 2020, 12, 1239–1255. [Google Scholar] [CrossRef] [PubMed]
- Thumfart, K.M.; Jawaid, A.; Bright, K.; Flachsmann, M.; Mansuy, I.M. Epigenetics of childhood trauma: Long term sequelae and potential for treatment. Neurosci. Biobehav. Rev. 2021, 132, 1049–1066. [Google Scholar] [CrossRef]
- Aisa, B.; Tordera, R.; Lasheras, B.; Del Río, J.; Ramírez, M.J. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology 2007, 32, 256–266. [Google Scholar] [CrossRef]
- Kalinichev, M.; Easterling, K.W.; Plotsky, P.M.; Holtzman, S.G. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long–Evans rats. Pharmacol. Biochem. Behav. 2002, 73, 131–140. [Google Scholar] [CrossRef]
- Lehmann, J.; Feldon, J. Long-term biobehavioral effects of maternal separation in the rat: Consistent or confusing? Rev. Neurosci. 2000, 11, 383–408. [Google Scholar] [CrossRef]
- Winters, K.C.; Arria, A. Adolescent brain development and drugs. Prev. Res. 2011, 18, 21. [Google Scholar]
- De Villiers, M.; Van den Berg, H. The implementation and evaluation of a resiliency programme for children. S. Afr. J. Psychol. 2012, 42, 93–102. [Google Scholar] [CrossRef]
- Scientific Council, N. Excessive stress disrupts the development of brain architecture. J. Child. Serv. 2014, 9, 143–153. [Google Scholar] [CrossRef]
- Liu, C.; Xu, L.; Li, J.; Zhou, F.; Yang, X.; Zheng, X.; Fu, M.; Li, K.; Sindermann, C.; Montag, C. Serotonin and early life stress interact to shape brain architecture and anxious avoidant behavior–a TPH2 imaging genetics approach. Psychol. Med. 2021, 51, 2476–2484. [Google Scholar] [CrossRef] [PubMed]
- Potter, M. History of the BALB/c family. In The BALB/c Mouse; Springer: Berlin/Heidelberg, Germany, 1985; pp. 1–5. [Google Scholar]
- Brodkin, E.S. BALB/c mice: Low sociability and other phenotypes that may be relevant to autism. Behav. Brain Res. 2007, 176, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Razzoli, M.; Carboni, L.; Andreoli, M.; Ballottari, A.; Arban, R. Different susceptibility to social defeat stress of BalbC and C57BL6/J mice. Behav. Brain Res. 2011, 216, 100–108. [Google Scholar] [CrossRef]
- Savignac, H.M.; Finger, B.C.; Pizzo, R.C.; O’leary, O.F.; Dinan, T.G.; Cryan, J.F. Increased sensitivity to the effects of chronic social defeat stress in an innately anxious mouse strain. Neuroscience 2011, 192, 524–536. [Google Scholar] [CrossRef] [Green Version]
- Kudryashov, N.V.; Kalinina, T.S.; Zhmurenko, L.A.; Voronina, T.A. Anticompulsive activity of a new pyrazolo [C] pyridine derivative GIZh-72 under conditions of unpredictable chronic mild stress. Bull. Exp. Biol. Med. 2016, 161, 377–380. [Google Scholar] [CrossRef]
- Gomes, J.A.S.; Oliveira, M.C.; Gobira, P.H.; Silva, G.C.; Marçal, A.P.; Gomes, G.F.; Ferrari, C.Z.; Lemos, V.S.; de Oliveira, A.C.P.; Vieira, L.B. A high-refined carbohydrate diet facilitates compulsive-like behavior in mice through the nitric oxide pathway. Nitric Oxide 2018, 80, 61–69. [Google Scholar] [CrossRef]
- Ponzoni, L.; Braida, D.; Carboni, L.; Moretti, M.; Viani, P.; Clementi, F.; Zoli, M.; Gotti, C.; Sala, M. Persistent cognitive and affective alterations at late withdrawal stages after long-term intermittent exposure to tobacco smoke or electronic cigarette vapour: Behavioural changes and their neurochemical correlates. Pharmacol. Res. 2020, 158, 104941. [Google Scholar] [CrossRef]
- Marchette, R.C.N.; Bicca, M.A.; da Silva Santos, E.C.; de Lima, T.C.M. Distinctive stress sensitivity and anxiety-like behavior in female mice: Strain differences matter. Neurobiol. Stress 2018, 9, 55–63. [Google Scholar] [CrossRef]
- Biedermann, S.V.; Biedermann, D.G.; Wenzlaff, F.; Kurjak, T.; Nouri, S.; Auer, M.K.; Wiedemann, K.; Briken, P.; Haaker, J.; Lonsdorf, T.B. An elevated plus-maze in mixed reality for studying human anxiety-related behavior. BMC Biol. 2017, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- Anggreini, P.; Ardianto, C.; Rahmadi, M.; Khotib, J. Quercetin attenuates acute predator stress exposure-evoked innate fear and behavioral perturbation. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Dalvi, A. Anxiety, defence and the elevated plus-maze. Neurosci. Biobehav. Rev. 1997, 21, 801–810. [Google Scholar] [CrossRef]
- Lalonde, R.; Strazielle, C. Relations between open-field, elevated plus-maze, and emergence tests in C57BL/6J and BALB/c mice injected with GABA-and 5HT-anxiolytic agents. Fundam. Clin. Pharmacol. 2010, 24, 365–376. [Google Scholar] [CrossRef]
- Brown, G.R.; Nemes, C. The exploratory behaviour of rats in the hole-board apparatus: Is head-dipping a valid measure of neophilia? Behav. Process. 2008, 78, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Bath, K.G.; Manzano-Nieves, G.; Goodwill, H. Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Horm. Behav. 2016, 82, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Bakhshani, N.-M. Impulsivity: A predisposition toward risky behaviors. Int. J. High Risk Behav. Addict. 2014, 3, e20428. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, D.M.; MacLennan, A.J.; Pinel, J.P.J. RAT Defensive Behavior: Burying Noxious Food 1. J. Exp. Anal. Behav. 1979, 31, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poling, A.; Cleary, J.; Monaghan, M. Burying by rats in response to aversive and nonaversive stimuli. J. Exp. Anal. Behav. 1981, 35, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Taylor, G.T.; Lerch, S.; Chourbaji, S. Marble burying as compulsive behaviors in male and female mice. Acta Neurobiol. Exp. 2017, 77, 254–260. [Google Scholar] [CrossRef] [Green Version]
- Llaneza, D.C.; Frye, C.A. Progestogens and estrogen influence impulsive burying and avoidant freezing behavior of naturally cycling and ovariectomized rats. Pharmacol. Biochem. Behav. 2009, 93, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cservenka, A.; Ray, L.A. Self-reported attentional and motor impulsivity are related to age at first methamphetamine use. Addict. Behav. 2017, 65, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Boger, S.; Ehring, T.; Schwarzkopf, W.; Werner, G.G. Potential mediators of the association between childhood maltreatment and obsessive-compulsive disorder in adulthood. J. Obs. Compuls. Relat. Disord. 2020, 27, 100587. [Google Scholar] [CrossRef]
- Bowlby, J. The bowlby-ainsworth attachment theory. Behav. Brain Sci. 1979, 2, 637–638. [Google Scholar] [CrossRef]
- Tops, M.; Van Peer, J.M.; Korf, J.; Wijers, A.A.; Tucker, D.M. Anxiety, cortisol, and attachment predict plasma oxytocin. Psychophysiology 2007, 44, 444–449. [Google Scholar] [CrossRef]
- De Weerth, C.; Buitelaar, J.K.; Beijers, R. Infant cortisol and behavioral habituation to weekly maternal separations: Links with maternal prenatal cortisol and psychosocial stress. Psychoneuroendocrinology 2013, 38, 2863–2874. [Google Scholar] [CrossRef] [Green Version]
- De Weerth, C.; Zijlmans, M.A.C.; Mack, S.; Beijers, R. Cortisol reactions to a social evaluative paradigm in 5-and 6-year-old children. Stress 2013, 16, 65–72. [Google Scholar] [CrossRef]
- Doulougeri, K.; Panagopoulou, E.; Montgomery, A. The impact of maternal stress on initiation and establishment of breastfeeding. J. Neonatal Nurs. 2013, 19, 162–167. [Google Scholar] [CrossRef]
- Fallon, V.; Groves, R.; Halford, J.C.G.; Bennett, K.M.; Harrold, J.A. Postpartum anxiety and infant-feeding outcomes: A systematic review. J. Hum. Lact. 2016, 32, 740–758. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarrar, Q.; Ayoub, R.; Alhussine, K.; Goh, K.W.; Moshawih, S.; Ardianto, C.; Goh, B.H.; Ming, L.C. Prolonged Maternal Separation Reduces Anxiety State and Increases Compulsive Burying Activity in the Offspring of BALB/c Mice. J. Pers. Med. 2022, 12, 1921. https://doi.org/10.3390/jpm12111921
Jarrar Q, Ayoub R, Alhussine K, Goh KW, Moshawih S, Ardianto C, Goh BH, Ming LC. Prolonged Maternal Separation Reduces Anxiety State and Increases Compulsive Burying Activity in the Offspring of BALB/c Mice. Journal of Personalized Medicine. 2022; 12(11):1921. https://doi.org/10.3390/jpm12111921
Chicago/Turabian StyleJarrar, Qais, Rami Ayoub, Kawther Alhussine, Khang Wen Goh, Said Moshawih, Chrismawan Ardianto, Bey Hing Goh, and Long Chiau Ming. 2022. "Prolonged Maternal Separation Reduces Anxiety State and Increases Compulsive Burying Activity in the Offspring of BALB/c Mice" Journal of Personalized Medicine 12, no. 11: 1921. https://doi.org/10.3390/jpm12111921