Association of Breast Tumour Expression of Cannabinoid Receptors CBR1 and CBR2 with Prognostic Factors and Survival in Breast Cancer Patients
Abstract
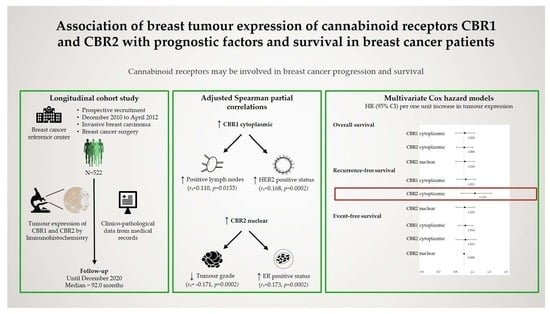
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Assessment of CBR Expression
2.4. Survival Outcome
2.5. Statistical Analyses
3. Results
3.1. Study Population
3.2. CBR Expression and Breast Cancer Prognostic Factors
3.3. CBR Expression and Breast Cancer Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pacher, P.; Bátkai, S.; Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blazquez, C.; Casanova, M.L.; Planas, A.; Gomez Del Pulgar, T.; Villanueva, C.; Fernandez-Acenero, M.J.; Aragones, J.; Huffman, J.W.; Jorcano, J.L.; Guzman, M. Inhibition of tumor angiogenesis by cannabinoids. FASEB J. 2003, 17, 529–531. [Google Scholar] [CrossRef]
- Mackie, K. Cannabinoid receptors: Where they are and what they do. J. Neuroendocrinol. 2008, 20 (Suppl. 1), 10–14. [Google Scholar] [CrossRef] [PubMed]
- Caffarel, M.M.; Sarrio, D.; Palacios, J.; Guzman, M.; Sanchez, C. Delta9-tetrahydrocannabinol inhibits cell cycle progression in human breast cancer cells through Cdc2 regulation. Cancer Res. 2006, 66, 6615–6621. [Google Scholar] [CrossRef] [Green Version]
- Moreno, E.; Cavic, M.; Krivokuca, A.; Casadó, V.; Canela, E. The Endocannabinoid System as a Target in Cancer Diseases: Are We There Yet? Front. Pharmacol. 2019, 10, 339. [Google Scholar] [CrossRef] [Green Version]
- Velasco, G.; Sánchez, C.; Guzmán, M. Anticancer mechanisms of cannabinoids. Curr. Oncol. 2016, 23, S23–S32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzman, M.; Sanchez, C.; Galve-Roperh, I. Cannabinoids and cell fate. Pharmacol. Ther. 2002, 95, 175–184. [Google Scholar] [CrossRef]
- Velasco, G.; Galve-Roperh, I.; Sanchez, C.; Blazquez, C.; Guzman, M. Hypothesis: Cannabinoid therapy for the treatment of gliomas? Neuropharmacology 2004, 47, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Smith, P.F.; Rosengren, R.J. Cannabinoids in the treatment of cancer. Cancer Lett. 2009, 285, 6–12. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Munson, A.E.; Harris, L.S.; Friedman, M.A. Antineoplastic activity of cannabinoids. J. Natl. Cancer Inst. 1975, 55, 597–602. [Google Scholar] [CrossRef]
- Galve-Roperh, I.; Sánchez, C.; Cortés, M.L.; Gómez del Pulgar, T.; Izquierdo, M.; Guzmán, M. Anti-tumoral action of cannabinoids: Involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 2000, 6, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; de Ceballos, M.L.; Gomez del Pulgar, T.; Rueda, D.; Corbacho, C.; Velasco, G.; Galve-Roperh, I.; Huffman, J.W.; Ramón y Cajal, S.; Guzmán, M. Inhibition of glioma growth in vivo by selective activation of the CB2 cannabinoid receptor. Cancer Res. 2001, 61, 5784–5789. [Google Scholar] [PubMed]
- Bifulco, M.; Laezza, C.; Portella, G.; Vitale, M.; Orlando, P.; De Petrocellis, L.; Di Marzo, V. Control by the endogenous cannabinoid system of ras oncogene-dependent tumor growth. FASEB J. 2001, 15, 2745–2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thors, L.; Bergh, A.; Persson, E.; Hammarsten, P.; Stattin, P.; Egevad, L.; Granfors, T.; Fowler, C.J. Fatty acid amide hydrolase in prostate cancer: Association with disease severity and outcome, CB1 receptor expression and regulation by IL-4. PLoS ONE 2010, 5, e12275. [Google Scholar] [CrossRef]
- Chung, S.C.; Hammarsten, P.; Josefsson, A.; Stattin, P.; Granfors, T.; Egevad, L.; Mancini, G.; Lutz, B.; Bergh, A.; Fowler, C.J. A high cannabinoid CB1 receptor immunoreactivity is associated with disease severity and outcome in prostate cancer. Eur. J. Cancer 2009, 45, 174–182. [Google Scholar] [CrossRef]
- Fowler, C.J.; Hammarsten, P.; Bergh, A. Tumour Cannabinoid CB1 receptor and phosphorylated epidermal growth factor receptor expression are additive prognostic markers for prostate cancer. PLoS ONE 2010, 5, e15205. [Google Scholar] [CrossRef]
- Martinez-Martinez, E.; Gomez, I.; Martin, P.; Sanchez, A.; Roman, L.; Tejerina, E.; Bonilla, F.; Merino, A.G.; de Herreros, A.G.; Provencio, M.; et al. Cannabinoids receptor type 2, CB2, expression correlates with human colon cancer progression and predicts patient survival. Oncoscience 2015, 2, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.K.; Kang, W.K.; Park, J.M.; Ahn, H.J.; Kim, S.W.; Oh, S.T.; Choi, K.Y. Expression of the cannabinoid type I receptor and prognosis following surgery in colorectal cancer. Oncol. Lett. 2013, 5, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, S.B.; Palmqvist, R.; Henriksson, M.L.; Dahlin, A.M.; Edin, S.; Jacobsson, S.O.; Oberg, A.; Fowler, C.J. High tumour cannabinoid CB1 receptor immunoreactivity negatively impacts disease-specific survival in stage II microsatellite stable colorectal cancer. PLoS ONE 2011, 6, e23003. [Google Scholar] [CrossRef]
- Zeng, C.; Chen, Y. HTR1D, TIMP1, SERPINE1, MMP3 and CNR2 affect the survival of patients with colon adenocarcinoma. Oncol. Lett. 2019, 18, 2448–2454. [Google Scholar] [CrossRef] [PubMed]
- Caffarel, M.M.; Andradas, C.; Perez-Gomez, E.; Guzman, M.; Sanchez, C. Cannabinoids: A new hope for breast cancer therapy? Cancer Treat. Rev. 2012, 38, 911–918. [Google Scholar] [CrossRef]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef] [Green Version]
- Qamri, Z.; Preet, A.; Nasser, M.W.; Bass, C.E.; Leone, G.; Barsky, S.H.; Ganju, R.K. Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol. Cancer Ther. 2009, 8, 3117–3129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ligresti, A.; Moriello, A.S.; Starowicz, K.; Matias, I.; Pisanti, S.; De Petrocellis, L.; Laezza, C.; Portella, G.; Bifulco, M.; Di Marzo, V. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J. Pharmacol. Exp. Ther. 2006, 318, 1375–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Gomez, E.; Andradas, C.; Blasco-Benito, S.; Caffarel, M.M.; Garcia-Taboada, E.; Villa-Morales, M.; Moreno, E.; Hamann, S.; Martin-Villar, E.; Flores, J.M.; et al. Role of cannabinoid receptor CB2 in HER2 pro-oncogenic signaling in breast cancer. J. Natl. Cancer Inst. 2015, 107, djv077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furrer, D.; Jacob, S.; Caron, C.; Sanschagrin, F.; Provencher, L.; Diorio, C. Tissue Microarray Is a Reliable Tool for the Evaluation of HER2 Amplification in Breast Cancer. Anticancer Res. 2016, 36, 4661–4666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, E.A.; Romond, E.H.; Suman, V.J.; Jeong, J.H.; Sledge, G.; Geyer, C.E., Jr.; Martino, S.; Rastogi, P.; Gralow, J.; Swain, S.M.; et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 2014, 32, 3744–3752. [Google Scholar] [CrossRef]
- Fitzgibbons, P.L.; Murphy, D.A.; Hammond, M.E.H.; Allred, D.C.; Valenstein, P.N. Recommendations for Validating Estrogen and Progesterone Receptor Immunohistochemistry Assays. Arch. Pathol. Lab. Med. 2010, 134, 930–935. [Google Scholar] [CrossRef]
- American Joint Committee on Cancer. Breast, 8th ed.; Springer: New York, NY, USA, 2018. [Google Scholar]
- Wang, C.; Li, Y.; Chen, H.; Huang, K.; Liu, X.; Qiu, M.; Liu, Y.; Yang, Y.; Yang, J. CYP4X1 Inhibition by Flavonoid CH625 Normalizes Glioma Vasculature through Reprogramming TAMs via CB2 and EGFR-STAT3 Axis. J. Pharmacol. Exp. Ther. 2018, 365, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, S.; Giaginis, C.; Alexandrou, P.; Rodriguez, J.; Tasoulas, J.; Danas, E.; Patsouris, E.; Klijanienko, J. Evaluation of cannabinoid CB1 and CB2 receptors expression in mobile tongue squamous cell carcinoma: Associations with clinicopathological parameters and patients’ survival. Tumour Biol. 2016, 37, 3647–3656. [Google Scholar] [CrossRef] [PubMed]
- VanderWeele, T.J.; Shpitser, I. A new criterion for confounder selection. Biometrics 2011, 67, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Lagerlund, M.; Bellocco, R.; Karlsson, P.; Tejler, G.; Lambe, M. Socio-economic factors and breast cancer survival—A population-based cohort study (Sweden). Cancer Causes Control 2005, 16, 419–430. [Google Scholar] [CrossRef]
- Herndon, J.E., 2nd; Kornblith, A.B.; Holland, J.C.; Paskett, E.D. Effect of socioeconomic status as measured by education level on survival in breast cancer clinical trials. Psychooncology 2013, 22, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Gong, Y.; Jiang, C.C.; Hu, X.; Di, G.H.; Shao, Z.M. Association between socioeconomic factors at diagnosis and survival in breast cancer: A population-based study. Cancer Med. 2020, 9, 1922–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrick, M.E.; Wightman, P.; Schoeni, R.F.; Schulenberg, J.E. Socioeconomic status and substance use among young adults: A comparison across constructs and drugs. J. Stud. Alcohol Drugs 2012, 73, 772–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, G.C.K.; Leung, J.; Quinn, C.; Weier, M.; Hall, W. Socio-economic differentials in cannabis use trends in Australia. Addiction 2018, 113, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Hango, D.; LaRochelle-Côté, S. Association between the Frequency of Cannabis Use and Selected Social Indicators. Insights on Canadian Society; Statistics Canada Catalogue no 75-006-X May 2018; Statistics Canada: Ottawa, ON, Canada, 2018; p. 20. [Google Scholar]


| Characteristics * | All (n = 522) | CBR1 (n = 489) | CBR2 in Cytoplasm (n = 475) | CBR2 in Nuclear (n = 475) | |||
|---|---|---|---|---|---|---|---|
| Low * (n = 235) | High * (n = 254) | Low * (n = 211) | High * (n = 264) | Low * (n = 206) | High * (n = 269) | ||
| Age (years) | |||||||
| Mean ± SD | 61.2 ± 12.6 | 62.3 ± 12.6 | 60.4 ± 12.99 | 62.9 ± 12.6 | 60.0 ± 12.8 | 61.9 ± 13.3 | 60.8 ± 12.3 |
| Median [range] | 61.0 [24.0–92.0] | 63.0 [32.0–91.0] | 61.0 [24.0–92.0] | 64.0 [24.0–91.0] | 61.0 [25.0–92.0] | 63.0 [24.0–91.0] | 61.0 [25.0–92.0] |
| Postmenopausal | 399 (76.4%) | 186 (79.2%) | 189 (74.4%) | 168 (79.6%) | 194 (73.5%) | 155 (75.2%) | 207 (77.0%) |
| Familial history of breast cancer (yes) | 245 (46.9%) | 110 (46.8%) | 123 (48.4%) | 97 (45.9%) | 125 (47.4%) | 96 (46.6%) | 126 (46.8%) |
| Ever smokers | 254 (48.7%) | 119 (50.6%) | 118 (46.5%) | 112 (53.1%) | 120 (45.5%) | 95 (46.1%) | 137 (50.9%) |
| Alcohol consumption (yes) | 337 (64.6%) | 147 (62.6%) | 168 (66.1%) | 128 (60.7%) | 179 (67.8%) | 131 (63.6%) | 176 (65.4%) |
| Body mass index (kg/m2) | |||||||
| Mean ± SD | 26.3 ± 5.2 | 25.8 ± 5.2 | 26.6 ± 5.3 | 26.1 ± 5.0 | 26.4 ± 5.5 | 26.3 ± 5.2 | 26.2 ± 5.3 |
| Median [range] | 25.5 [15.2–45.9] | 24.6 [16.3–44.1] | 25.6 [15.2–45.9] | 25.0 [17.7–44.1] | 25.5 [15.2–45.9] | 25.6 [16.3–44.6] | 25.3 [15.2–45.9] |
| Personal history of breast cancer (yes) | 63 (12.1%) | 25 (10.6%) | 32 (12.6%) | 26 (12.3%) | 29 (11.0%) | 23 (11.2%) | 32 (11.9%) |
| Histologic type | |||||||
| Ductal, invasive | 451 (86.4%) | 204 (86.8%) | 220 (86.6%) | 180 (85.3%) | 235 (89.0%) | 189 (91.8%) | 226 (84.0%) |
| Lobular, invasive | 56 (10.7%) | 27 (11.5%) | 24 (9.5%) | 24 (11.4%) | 23 (8.7%) | 12 (5.8%) | 35 (13.0%) |
| Mixed ductal and lobular, invasive | 15 (2.9%) | 4 (1.7%) | 10 (3.9%) | 7 (3.3%) | 6 (2.3%) | 5 (2.4%) | 8 (3.0%) |
| Tumour grade | |||||||
| 1 | 86 (16.5%) | 39 (16.6%) | 38 (15.0%) | 30 (14.2%) | 42 (18.9%) | 23 (11.2%) | 49 (18.2%) |
| 2 | 258 (49.4%) | 112 (47.7%) | 130 (51.2%) | 119 (56.4%) | 116 (43.9%) | 90 (43.7%) | 145 (53.9%) |
| 3 | 178 (34.1%) | 84 (35.7%) | 86 (33.9%) | 62 (29.4%) | 106 (40.2%) | 93 (45.2%) | 75 (27.9%) |
| Tumour size | |||||||
| ≤2 cm | 293 (56.1%) | 130 (55.3%) | 140 (55.1%) | 114 (54.0%) | 148 (56.1%) | 110 (53.4%) | 152 (56.5%) |
| >2 and ≤5 cm | 209 (40.0%) | 101 (43.0%) | 99 (39.0%) | 89 (42.2%) | 106 (40.2%) | 93 (45.2%) | 102 (37.9%) |
| >5 cm | 20 (3.8%) | 4 (1.7%) | 15 (5.9%) | 8 (3.8%) | 10 (3.8%) | 3 (1.5%) | 15 (5.6%) |
| Positive lymph nodes | |||||||
| 0 | 312 (59.8%) | 153 (65.1%) | 137 (53.9%) | 135 (64.0%) | 146 (55.3%) | 125 (60.7%) | 156 (58.0%) |
| 1–3 | 146 (28.0%) | 60 (25.5%) | 78 (30.7%) | 55 (26.1%) | 80 (30.3%) | 59 (28.6%) | 76 (28.3%) |
| 4–9 | 42 (8.0%) | 17 (7.2%) | 22 (8.7%) | 14 (6.6%) | 26 (9.8%) | 18 (8.7%) | 22 (8.2%) |
| ≥10 | 22 (4.2%) | 5 (2.1%) | 17 (6.7%) | 7 (3.3%) | 12 (4.6%) | 4 (1.9%) | 15 (5.6%) |
| Disease stage | |||||||
| I | 203 (38.9%) | 96 (40.9%) | 91 (35.8%) | 84 (39.8%) | 97 (36.7%) | 76 (36.9%) | 105 (39.0%) |
| II | 241 (46.2%) | 110 (46.8%) | 119 (46.9%) | 101 (47.9%) | 122 (46.2%) | 104 (50.5%) | 119 (44.2%) |
| III | 71 (13.6%) | 28 (11.9%) | 38 (18.0%) | 25 (11.9%) | 39 (14.8%) | 24 (11.7%) | 40 (14.9%) |
| IV | 7 (1.3%) | 1 (0.4%) | 6 (2.4%) | 1 (0.5%) | 6 (2.3%) | 2 (1.0%) | 5 (1.9%) |
| ER status | |||||||
| Negative | 64 (12.3%) | 29 (12.3%) | 30 (11.8%) | 35 (16.6%) | 25 (9.5%) | 37 (18.0%) | 23 (8.6%) |
| Positive | 458 (87.7%) | 206 (87.7%) | 224 (88.2%) | 176 (83.4%) | 239 (90.5%) | 169 (82.0%) | 246 (91.5%) |
| PR status | |||||||
| Negative | 108 (20.7%) | 46 (19.6%) | 52 (20.5%) | 51 (24.1%) | 47 (17.8%) | 53 (25.7%) | 45 (16.7%) |
| Positive | 414 (79.3%) | 189 (80.4%) | 202 (79.5%) | 160 (75.8%) | 217 (82.2%) | 153 (74.3%) | 224 (83.3%) |
| HER2 status | |||||||
| Negative | 455 (87.2%) | 215 (91.5%) | 210 (82.7%) | 191 (90.5%) | 222 (84.1%) | 180 (87.4%) | 233 (86.6%) |
| Positive | 67 (12.8%) | 20 (8.5%) | 44 (17.3%) | 20 (9.5%) | 42 (15.9%) | 26 (12.6%) | 36 (13.4%) |
| Surgery | |||||||
| Partial | 384 (73.6%) | 173 (73.6%) | 187 (73.6%) | 156 (73.9%) | 195 (73.9%) | 154 (74.8%) | 197 (73.2%) |
| Total | 138 (26.4%) | 62 (26.4%) | 67 (26.4%) | 55 (26.1%) | 69 (26.1%) | 52 (25.2%) | 72 (26.8%) |
| Chemotherapy prior to surgery (yes) | 6 (1.2%) | 1 (0.4%) | 2 (0.8%) | 1 (0.5%) | 2 (0.8%) | 1 (0.5%) | 2 (0.7%) |
| Hormone therapy prior to surgery (yes) | 6 (1.2%) | 3 (1.3%) | 3 (1.2%) | 4 (1.9%) | 2 (0.8%) | 1 (0.5%) | 5 (1.9%) |
| Chemotherapy after surgery (yes) | 263 (50.4%) | 108 (46.0%) | 138 (54.3%) | 94 (44.6%) | 146 (55.3%) | 106 (51.5%) | 134 (49.8%) |
| Radiotherapy after surgery (yes) | 414 (79.3%) | 184 (78.3%) | 204 (80.3%) | 164 (77.7%) | 212 (80.3%) | 163 (79.1%) | 213 (79.2%) |
| Hormone therapy after surgery (yes) | 433 (83.0%) | 189 (80.4%) | 217 (85.4%) | 164 (77.7%) | 229 (86.7%) | 157 (76.2%) | 236 (87.7%) |
| Trastuzumab after surgery (yes) | 59 (11.3%) | 20 (8.4%) | 36 (14.2%) | 15 (7.1%) | 39 (14.8%) | 23 (11.2%) | 31 (11.5%) |
| Follow-up (months) | |||||||
| Mean ± SD | 84.7 ± 20.7 | 83.0 ± 21.7 | 86.3 ± 19.6 | 84.6 ± 20.8 | 84.4 ± 21.2 | 84.7 ± 21.2 | 84.7 ± 20.9 |
| Median [range] | 92.0 [7.0–114.0] | 91.0 [7.0–112.0] | 93.0 [11.0–114] | 93.0 [7.0–112.0] | 92.0 [7.0–114.0] | 92.0 [14.0–112.0] | 92.0 [7.0–114.0] |
| Survival outcomes | |||||||
| Deaths | 86 (16.5%) | 41 (17.5%) | 40 (15.8%) | 39 (18.5%) | 43 (16.3%) | 42 (20.4%) | 40 (14.9%) |
| Recurrences § | 74 (14.2%) | 31 (13.2%) | 36 (14.2%) | 24 (11.4%) | 44 (16.7%) | 31 (15.1%) | 37 (13.8%) |
| Events † | 143 (27.4%) | 65 (27.7%) | 69 (27.2%) | 60 (28.4%) | 74 (28.0%) | 65 (31.6%) | 69 (25.7%) |
| CBR1 (n = 489) | CBR2 in Cytoplasm (n = 475) | CBR2 in Nuclear (n = 475) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted * | Fully Adjusted § | Unadjusted | Adjusted * | Fully Adjusted § | Unadjusted | Adjusted * | Fully Adjusted § | |
| Histologic type | |||||||||
| rs | −0.009 | 0.004 | 0.006 | −0.022 | −0.004 | −0.002 | 0.144 | 0.148 | 0.147 |
| p-value | 0.8451 | 0.9316 | 0.9045 | 0.6399 | 0.9301 | 0.9602 | 0.0016 | 0.0013 | 0.0014 |
| Tumour size | |||||||||
| rs | 0.017 | 0.025 | 0.011 | 0.030 | 0.046 | 0.032 | 0.013 | 0.023 | 0.030 |
| p-value | 0.7148 | 0.5795 | 0.8074 | 0.5158 | 0.3207 | 0.4843 | 0.7721 | 0.6163 | 0.5163 |
| Positive lymph nodes | |||||||||
| rs | 0.116 | 0.110 | 0.107 | 0.072 | 0.063 | 0.060 | 0.060 | 0.063 | 0.064 |
| p-value | 0.0103 | 0.0155 | 0.0194 | 0.1180 | 0.1767 | 0.1978 | 0.2158 | 0.1770 | 0.1676 |
| Tumour grade | |||||||||
| rs | 0.003 | −0.006 | −0.014 | 0.052 | 0.043 | 0.036 | −0.170 | −0.171 | −0.170 |
| p-value | 0.9451 | 0.9008 | 0.7632 | 0.2572 | 0.3577 | 0.4420 | 0.0002 | 0.0002 | 0.0002 |
| Disease stage | |||||||||
| rs | 0.073 | 0.077 | 0.067 | 0.050 | 0.055 | 0.046 | 0.020 | 0.025 | 0.030 |
| p-value | 0.1083 | 0.0898 | 0.1444 | 0.2812 | 0.2310 | 0.3242 | 0.6603 | 0.5929 | 0.5227 |
| ER status | |||||||||
| rs | 0.028 | 0.021 | 0.022 | 0.096 | 0.089 | 0.090 | 0.172 | 0.173 | 0.173 |
| p-value | 0.5448 | 0.6405 | 0.6336 | 0.0358 | 0.0546 | 0.0528 | 0.0002 | 0.0002 | 0.0002 |
| PR status | |||||||||
| rs | −0.023 | −0.031 | −0.029 | 0.033 | 0.025 | 0.026 | 0.119 | 0.121 | 0.121 |
| p-value | 0.6081 | 0.5027 | 0.5287 | 0.4704 | 0.5963 | 0.5755 | 0.0096 | 0.0084 | 0.0087 |
| HER2 status | |||||||||
| rs | 0.176 | 0.168 | 0.165 | 0.086 | 0.080 | 0.076 | 0.031 | 0.032 | 0.034 |
| p-value | <0.0001 | 0.0002 | 0.0003 | 0.0623 | 0.0848 | 0.0990 | 0.4989 | 0.4915 | 0.4695 |
| CBR | Events/Total | Crude HR (95% CI) | p-Value | Adjusted § HR (95% CI) | p-Value | Fully Adjusted † HR (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|
| Overall survival | |||||||
| CBR1 | 81/489 | 0.98 [0.88–1.09] | 0.70 | 1.01 [0.90–1.13] | 0.86 | 1.02 [0.91–1.14] | 0.74 |
| CBR2 cytoplasmic | 82/475 | 0.95 [0.82–1.09] | 0.46 | 1.03 [0.88–1.20] | 0.74 | 1.02 [0.87–1.19] | 0.84 |
| CBR2 nuclear | 82/475 | 0.99 [0.90–1.09] | 0.76 | 1.01 [0.90–1.11] | 0.91 | 1.01 [0.92–1.12] | 0.81 |
| Recurrence-free survival | |||||||
| CBR1 | 67/489 | 1.04 [0.93–1.17] | 0.49 | 1.02 [0.92–1.14] | 0.71 | 1.04 [0.93–1.18] | 0.49 |
| CBR2 cytoplasmic | 68/475 | 0.96 [0.83–1.11] | 0.59 | 1.13 [0.97–1.33] | 0.13 | 1.09 [0.93–1.28] | 0.28 |
| CBR2 nuclear | 68/475 | 0.99 [0.90–1.09] | 0.77 | 1.01 [0.90–1.13] | 0.86 | 1.01 [0.90–1.13] | 0.86 |
| Event-free survival | |||||||
| CBR1 | 134/489 | 1.00 [0.92–1.09] | 0.97 | 1.01 [0.93–1.10] | 0.75 | 1.01 [0.93–1.10] | 0.81 |
| CBR2 cytoplasmic | 134/475 | 0.98 [0.87–1.10] | 0.71 | 1.01 [0.90–1.14] | 0.81 | 1.01 [0.90–1.14] | 0.82 |
| CBR2 nuclear | 134/475 | 0.98 [0.91–1.06] | 0.69 | 1.00 [1.00–1.00] | 0.22 | 1.00 [1.00–1.00] | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morin-Buote, J.; Ennour-Idrissi, K.; Poirier, É.; Lemieux, J.; Furrer, D.; Burguin, A.; Durocher, F.; Diorio, C. Association of Breast Tumour Expression of Cannabinoid Receptors CBR1 and CBR2 with Prognostic Factors and Survival in Breast Cancer Patients. J. Pers. Med. 2021, 11, 852. https://doi.org/10.3390/jpm11090852
Morin-Buote J, Ennour-Idrissi K, Poirier É, Lemieux J, Furrer D, Burguin A, Durocher F, Diorio C. Association of Breast Tumour Expression of Cannabinoid Receptors CBR1 and CBR2 with Prognostic Factors and Survival in Breast Cancer Patients. Journal of Personalized Medicine. 2021; 11(9):852. https://doi.org/10.3390/jpm11090852
Chicago/Turabian StyleMorin-Buote, Jessica, Kaoutar Ennour-Idrissi, Éric Poirier, Julie Lemieux, Daniela Furrer, Anna Burguin, Francine Durocher, and Caroline Diorio. 2021. "Association of Breast Tumour Expression of Cannabinoid Receptors CBR1 and CBR2 with Prognostic Factors and Survival in Breast Cancer Patients" Journal of Personalized Medicine 11, no. 9: 852. https://doi.org/10.3390/jpm11090852