Molecular Prognostic Factors for Distant Metastases in Premenopausal Patients with HR+/HER2− Early Breast Cancer
Abstract
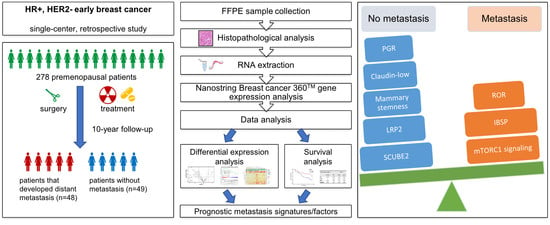
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethics Approval and Consent
2.3. Gene Expression Profiling
2.4. Gene and Signature Expression Analysis
2.5. Differential Expression Analysis
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics and Treatment
3.2. Expression Profiles of Premenopausal HR+/HER2− EBC with Subsequent Metastasis Are Associated with More Aggressive Molecular Subtypes
3.3. Increased ROR Scores and MTORC1 Signaling as Well as Reduced Claudin-Low, Mammary Stemness and PGR Signatures Are Associated with Premenopausal EBC with Subsequent Metastasis
3.4. SCUBE2, LRP2, and IBSP Are Independent Prognostic Markers of Subsequent Metastasis of Premenopausal HR+/HER2− EBC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- He, Z.; Chen, Z.; Tan, M.; Elingarami, S.; Liu, Y.; Li, T.; Deng, Y.; He, N.; Li, S.; Fu, J.; et al. A review on methods for diagnosis of breast cancer cells and tissues. Cell Prolif. 2020, 53, e12822. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.E.R. Precision Medicine for Breast Cancer: The Paths to Truly Individualized Diagnosis and Treatment. Int. J. Breast Cancer 2018, 2018, 4809183. [Google Scholar] [CrossRef] [Green Version]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andre, F.; Ismaila, N.; Henry, N.L.; Somerfield, M.R.; Bast, R.C.; Barlow, W.; Collyar, D.E.; Hammond, M.E.; Kuderer, N.M.; Liu, M.C.; et al. Use of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women With Early-Stage Invasive Breast Cancer: ASCO Clinical Practice Guideline Update-Integration of Results From TAILORx. J. Clin. Oncol. 2019, 85, 402–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burstein, H.J.; Curigliano, G.; Loibl, S.; Dubsky, P.; Gnant, M.; Poortmans, P.; Colleoni, M.; Denkert, C.; Piccart-Gebhart, M.; Regan, M.; et al. Estimating the benefits of therapy for early-stage breast cancer: The St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann. Oncol. 2019, 30, 1541–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, R.; Tyson, J.J.; Dixon, J.M. Metastatic behavior of breast cancer subtypes. Mol. Cell. Endocrinol. 2015, 418, 220–234. [Google Scholar] [CrossRef] [Green Version]
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 2010, 28, 3271–3277. [Google Scholar] [CrossRef]
- Kim, E.K.; Noh, W.C.; Han, W.; Noh, D.Y. Prognostic significance of young age (<35 years) by subtype based on ER, PR, and HER2 status in breast cancer: A nationwide registry-based study. World J. Surg. 2011, 35, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Azim, H.A., Jr.; Davidson, N.E.; Ruddy, K.J. Challenges in Treating Premenopausal Women with Endocrine-Sensitive Breast Cancer. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, 23–32. [Google Scholar] [CrossRef]
- Ahn, S.H.; Son, B.H.; Kim, S.W.; Kim, S.I.; Jeong, J.; Ko, S.S.; Han, W. Korean Breast Cancer S. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: Nationwide survival data in Korea—A report from the Korean Breast Cancer Society. J. Clin. Oncol. 2007, 25, 2360–2368. [Google Scholar] [CrossRef]
- Suter, M.B.; Pagani, O. Should age impact breast cancer management in young women? Fine tuning of treatment guidelines. Ther. Adv. Med. Oncol. 2018, 10, 1758835918776923. [Google Scholar] [CrossRef] [PubMed]
- Anders, C.K.; Fan, C.; Parker, J.S.; Carey, L.A.; Blackwell, K.L.; Klauber-DeMore, N.; Perou, C.M. Breast carcinomas arising at a young age: Unique biology or a surrogate for aggressive intrinsic subtypes? J. Clin. Oncol. 2011, 29, e18–e20. [Google Scholar] [CrossRef] [Green Version]
- Anders, C.K.; Hsu, D.S.; Broadwater, G.; Acharya, C.R.; Foekens, J.A.; Zhang, Y.; Wang, Y.; Marcom, P.K.; Marks, J.R.; Febbo, P.G.; et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J. Clin. Oncol. 2008, 26, 3324–3330. [Google Scholar] [CrossRef] [Green Version]
- Liao, S.; Hartmaier, R.J.; McGuire, K.P.; Puhalla, S.L.; Luthra, S.; Chandran, U.R.; Ma, T.; Bhargava, R.; Modugno, F.; Davidson, N.E.; et al. The molecular landscape of premenopausal breast cancer. Breast Cancer Res. 2015, 17, 104. [Google Scholar] [CrossRef] [Green Version]
- Wallden, B.; Storhoff, J.; Nielsen, T.; Dowidar, N.; Schaper, C.; Ferree, S.; Liu, S.; Leung, S.; Geiss, G.; Snider, J.; et al. Development and verification of the PAM50-based Prosigna breast cancer gene signature assay. BMC Med. Genom. 2015, 8, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.; Han, Y.; He, Q. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.J.; Kuhn, M.; Stark, M.; Chaffron, S.; Creevey, C.; Muller, J.; Doerks, T.; Julien, P.; Roth, A.; Simonovic, M.; et al. STRING 8—A global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 2008, 37, D412–D416. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Győrffy, B. Survival analysis across the entire transcriptome identifies biomarkers with the highest prognostic power in breast cancer. Comput. Struct. Biotechnol. J. 2021, 19, 4101–4109. [Google Scholar] [CrossRef]
- Gnant, M.; Filipits, M.; Greil, R.; Stoeger, H.; Rudas, M.; Bago-Horvath, Z.; Mlineritsch, B.; Kwasny, W.; Knauer, M.; Singer, C.; et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: Using the PAM50 Risk of Recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann. Oncol. 2014, 25, 339–345. [Google Scholar] [CrossRef]
- Duffy, M.J.; Harbeck, N.; Nap, M.; Molina, R.; Nicolini, A.; Senkus, E.; Cardoso, F. Clinical use of biomarkers in breast cancer: Updated guidelines from the European Group on Tumor Markers (EGTM). Eur. J. Cancer 2017, 75, 284–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurozumi, S.; Matsumoto, H.; Hayashi, Y.; Tozuka, K.; Inoue, K.; Horiguchi, J.; Takeyoshi, I.; Oyama, T.; Kurosumi, M. Power of PgR expression as a prognostic factor for ER-positive/HER2-negative breast cancer patients at intermediate risk classified by the Ki67 labeling index. BMC Cancer 2017, 17, 354. [Google Scholar] [CrossRef]
- Prat, A.; Parker, J.S.; Karginova, O.; Fan, C.; Livasy, C.; Herschkowitz, J.I.; He, X.; Perou, C.M. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010, 12, R68. [Google Scholar] [CrossRef] [Green Version]
- Fougner, C.; Bergholtz, H.; Norum, J.H.; Sorlie, T. Re-definition of claudin-low as a breast cancer phenotype. Nat. Commun. 2020, 11, 1787. [Google Scholar] [CrossRef] [Green Version]
- Kotiyal, S.; Bhattacharya, S. Breast cancer stem cells, EMT and therapeutic targets. Biochem. Biophys. Res. Commun. 2014, 453, 112–116. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steelman, L.S.; Martelli, A.M.; Cocco, L.; Libra, M.; Nicoletti, F.; Abrams, S.L.; McCubrey, J.A. The therapeutic potential of mTOR inhibitors in breast cancer. Br. J. Clin. Pharmacol. 2016, 82, 1189–1212. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Breast Cancer; Version 4.2021; NCCN: Plymouth Meeting, PA, USA, 2021; p. 79. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 12 May 2021).
- Zhou, H.Y.; Huang, S.L. Current development of the second generation of mTOR inhibitors as anticancer agents. Chin. J. Cancer 2012, 31, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Loh, K.; Yap, Y.S. PI3K/Akt/mTOR inhibitors in breast cancer. Cancer Biol. Med. 2015, 12, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Herzog, K.; Willnow, T.E. LRP2, an auxiliary receptor that controls sonic hedgehog signaling in development and disease. Dev. Dyn. 2016, 245, 569–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonias, S.L.; Karimi-Mostowfi, N.; Murray, S.S.; Mantuano, E.; Gilder, A.S. Expression of LDL receptor-related proteins (LRPs) in common solid malignancies correlates with patient survival. PLoS ONE 2017, 12, e0186649. [Google Scholar] [CrossRef] [Green Version]
- Gordon, J.A.; Sodek, J.; Hunter, G.K.; Goldberg, H.A. Bone sialoprotein stimulates focal adhesion-related signaling pathways: Role in migration and survival of breast and prostate cancer cells. J. Cell Biochem. 2009, 107, 1118–1128. [Google Scholar] [CrossRef]
- Zhang, Y.; He, W.; Zhang, S. Seeking for Correlative Genes and Signaling Pathways With Bone Metastasis From Breast Cancer by Integrated Analysis. Front. Oncol. 2019, 9, 138. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.C.; Chen, C.C.; Cheng, C.J.; Yang, R.B. Domain and functional analysis of a novel breast tumor suppressor protein, SCUBE2. J. Biol. Chem. 2011, 286, 27039–27047. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.J.; Lin, Y.C.; Tsai, M.T.; Chen, C.S.; Hsieh, M.C.; Chen, C.L.; Yang, R.B. SCUBE2 suppresses breast tumor cell proliferation and confers a favorable prognosis in invasive breast cancer. Cancer Res. 2009, 69, 3634–3641. [Google Scholar] [CrossRef] [Green Version]




| Feature/Treatment | Groups | M0 (n = 49) | M1 (n = 48) | ||
|---|---|---|---|---|---|
| Age at diagnosis (years) | Median (range) | 47 (29–50) | 43 (30–50) | ||
| Tumor size (cm) | Median (range) | 1.7 (0.3–8.0) | 2.3 (0.2–6.3) | ||
| Side | left | 22 | 52.4% | 20 | 46.5% |
| right | 20 | 47.6% | 23 | 53.5% | |
| Grade | 1 | 6 | 12.2% | 1 | 2.1% |
| 2 | 31 | 63.3% | 26 | 54.2% | |
| 3 | 12 | 24.5% | 21 | 43.8% | |
| Histological type | ductal | 39 | 92.9% | 41 | 95.3% |
| lobular | 3 | 7.1% | 2 | 4.7% | |
| pT | 1 | 31 | 63.3% | 17 | 35.4% |
| 2 | 13 | 26.5% | 25 | 52.1% | |
| 3 | 5 | 10.2% | 6 | 12.5% | |
| pN | 0 | 32 | 65.3% | 13 | 27.1% |
| 1 | 11 | 22.4% | 19 | 39.6% | |
| 2 | 4 | 8.2% | 11 | 22.9% | |
| 3 | 2 | 4.1% | 5 | 10.4% | |
| Surgery | lumpectomy | 35 | 71.4% | 31 | 64.6% |
| mastectomy | 14 | 28.6% | 17 | 35.4% | |
| ALND | yes | 24 | 49.0% | 43 | 89.6% |
| no | 25 | 51.0% | 5 | 10.4% | |
| Radiotherapy | yes | 44 | 100.0% | 39 | 86.7% |
| no | 0 | 0.0% | 6 | 13.3% | |
| Chemotherapy | yes | 29 | 60.4% | 41 | 89.1% |
| no | 19 | 39.6% | 5 | 10.9% | |
| Taxane | yes | 11 | 47.8% | 23 | 62.2% |
| no | 12 | 52.2% | 14 | 37.8% | |
| Antracycline | yes | 22 | 100.0% | 32 | 86.5% |
| no | 0 | 0.0% | 5 | 13.5% | |
| Endocrine therapy | TAM | 33 | 75.0% | 31 | 81.6% |
| AI + GnRHa | 3 | 6.8% | 2 | 5.3% | |
| sequence of both | 8 | 18.2% | 5 | 13.2% | |
| Gene Symbol | Gene Description | M1 vs. M0 | Univariate Survival Analysis | ||||
|---|---|---|---|---|---|---|---|
| 95% CI | |||||||
| Log2 FC | p-value | HR | p-value | lower | upper | ||
| LRP2 | low-density lipoprotein receptor-related protein 2 | −1.54 | <0.001 | 0.693 | <0.001 | 0.583 | 0.823 |
| SFRP1 | secreted frizzled-related protein 1 | −1.29 | 0.001 | 0.749 | 0.002 | 0.625 | 0.899 |
| CDC14A | cell division cycle 14A | −0.71 | 0.002 | 0.623 | 0.005 | 0.448 | 0.867 |
| OGN | osteoglycin | −0.91 | 0.003 | 0.757 | 0.008 | 0.616 | 0.93 |
| ABCA8 | ATP binding cassette subfamily A member 8 | −0.71 | 0.004 | 0.697 | 0.005 | 0.54 | 0.899 |
| IGF1 | insulin-like growth factor 1 | −0.6 | 0.004 | 0.682 | 0.01 | 0.503 | 0.925 |
| BCAS1 | breast carcinoma amplified sequence 1 | 0.9 | 0.004 | 1.33 | 0.009 | 1.074 | 1.648 |
| IBSP | integrin binding sialoprotein | 0.62 | 0.006 | 1.495 | 0.006 | 1.122 | 1.992 |
| WNT11 | Wnt family member 11 | −0.71 | 0.006 | 0.705 | 0.01 | 0.537 | 0.928 |
| IRX1 | iroquois homeobox 1 | −0.91 | 0.01 | 0.767 | 0.01 | 0.625 | 0.94 |
| ERBB4 | erb-b2 receptor tyrosine kinase 4 | −0.66 | 0.01 | 0.803 | 0.03 | 0.661 | 0.975 |
| SOX10 | SRY-Box Transcription Factor 10 | −1.64 | 0.01 | 0.882 | 0.02 | 0.793 | 0.982 |
| MIA | melanoma inhibitory activity | −0.67 | 0.01 | 0.676 | 0.01 | 0.495 | 0.923 |
| PGR | Progesterone Receptor | −1.02 | 0.02 | 0.847 | 0.02 | 0.736 | 0.976 |
| HOXA5 | homeobox A5 | −0.6 | 0.02 | 0.74 | 0.04 | 0.56 | 0.979 |
| STC1 | stanniocalcin 1 | 0.79 | 0.02 | 1.187 | 0.052 | 0.998 | 1.412 |
| THBS4 | thrombospondin 4 | −0.62 | 0.03 | 0.791 | 0.048 | 0.627 | 0.998 |
| PTGER3 | prostaglandin E receptor 3 | −0.6 | 0.03 | 0.778 | 0.02 | 0.625 | 0.967 |
| SCUBE2 | Signal Peptide, CUB Domain, and EGF Like Domain Containing 2 | −0.76 | 0.03 | 0.844 | 0.04 | 0.716 | 0.994 |
| SFRP4 | secreted frizzled-related protein 4 | −0.59 | 0.04 | 0.82 | 0.07 | 0.66 | 1.019 |
| HSPA2 | Heat Shock Protein Family A Hsp70 Member 2 | −0.66 | 0.04 | 0.817 | 0.06 | 0.662 | 1.007 |
| ZBTB16 | Zinc Finger and BTB Domain Containing 16 | −0.73 | 0.04 | 0.822 | 0.04 | 0.68 | 0.993 |
| Gene Symbol | Affymetrix ID | Patients in Cohorts | HR (95% CI) | p-Value 1 |
|---|---|---|---|---|
| LRP2 | 205710_at | 2301 | 0.65 (0.54–0.77) | 4.6 × 10−7 |
| SFRP1 | 202035_s_at | 2301 | 0.67 (0.57–0.8) | 5.6 × 10−6 |
| CDC14A | 205288_at | 2301 | 0.91 (0.76–1.07) | 0.25 |
| OGN | 218730_s_at | 2301 | 0.69 (0.58–0.81) | 1.4 × 10−5 |
| ABCA8 | 204719_at | 2301 | 0.69 (0.58–0.82) | 2 × 10−5 |
| IGF1 | 209540_at | 2301 | 0.71 (0.6–0.84) | 8.3 × 10−5 |
| BCAS1 | 204378_at | 2301 | 0.99 (0.84–1.17) | 0.91 |
| IBSP | 207370_at | 2301 | 1.14 (0.96–1.35) | 0.13 |
| WNT11 | 206737_at | 2301 | 0.83 (0.7–0.98) | 0.032 |
| IRX1 | 230472_at | 764 | 0.67 (0.49–0.91) | 0.011 |
| ERBB4 | 206794_at | 2301 | 0.79 (0.66–0.93) | 0.0054 |
| SOX10 | 209842_at | 2301 | 0.71 (0.6–0.84) | 8.6 × 10−5 |
| MIA | 206560_s_at | 2301 | 0.68 (0.58–0.81) | 1.3 × 10−5 |
| PGR | 208305_at | 2301 | 0.71 (0.6–0.84) | 6.7 × 10−5 |
| HOXA5 | 213844_at | 2301 | 0.82 (0.69–0.97) | 0.02 |
| THBS4 | 204776_at | 2301 | 0.85 (0.72–1.01) | 0.062 |
| PTGER3 | 208169_s_at | 2301 | 0.81 (0.68–0.96) | 0.014 |
| SCUBE2 | 219197_s_at | 2301 | 0.66 (0.56–0.79) | 2.3 × 10−6 |
| ZBTB16 | 205883_at | 2301 | 0.68 (0.57–0.8) | 6.9 x 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, H.; Kumbrink, J.; Mayr, D.; Seiler, A.; Hagemann, F.; Degenhardt, T.; Sagebiel, S.; Würstlein, R.; Kates, R.; Harbeck, N.; et al. Molecular Prognostic Factors for Distant Metastases in Premenopausal Patients with HR+/HER2− Early Breast Cancer. J. Pers. Med. 2021, 11, 835. https://doi.org/10.3390/jpm11090835
Ni H, Kumbrink J, Mayr D, Seiler A, Hagemann F, Degenhardt T, Sagebiel S, Würstlein R, Kates R, Harbeck N, et al. Molecular Prognostic Factors for Distant Metastases in Premenopausal Patients with HR+/HER2− Early Breast Cancer. Journal of Personalized Medicine. 2021; 11(9):835. https://doi.org/10.3390/jpm11090835
Chicago/Turabian StyleNi, Hua, Jörg Kumbrink, Doris Mayr, Alina Seiler, Friederike Hagemann, Tom Degenhardt, Sabine Sagebiel, Rachel Würstlein, Ronald Kates, Nadia Harbeck, and et al. 2021. "Molecular Prognostic Factors for Distant Metastases in Premenopausal Patients with HR+/HER2− Early Breast Cancer" Journal of Personalized Medicine 11, no. 9: 835. https://doi.org/10.3390/jpm11090835