1. Introduction
Gliomas are common brain tumors that start in the glial cells, which are gluey supportive cells that surround nerve cells in the brain, and they represent 80% of primary malignant brain tumors [
1,
2]. These tumors can affect brain function and be life-threating depending on growth rate, size, and location. Glioma tumors are classified into two types according to their aggressiveness: low-grade glioma (LGG) and high-grade glioma (HGG) [
3,
4]. Some LGGs are benign tumors [
5], while HGGs are malignant tumors [
6]. Malignant tumors are aggressive tumors that contain cancerous cells and are life-threatening. These tumors have irregular boundaries with a high growth rate. Glioblastoma multiforme (GBM) tumors are the most common type of HGG [
3,
7,
8], classified as a grade IV glioma, the most aggressive grade of brain tumors, by the World Health Organization (WHO) [
2,
9,
10]. These tumors represent 50% of gliomas [
2,
11,
12]. Furthermore, 90% of GBMs are primary tumors [
9,
13]. Unfortunately, patients with GBM tumors have a very poor survival rate and prognosis outcome. Several risk factors are recognized to increase the chance of developing brain tumors. Some of these factors can be controlled and are related to the patient’s lifestyle behaviors, such as smoking, dietary habits, and alcohol intake. However, other factors cannot be controlled such as age, family history, and genetics. In addition, some diagnosis methods that are based on ionizing radiation, such as prenatal diagnostic X-ray exposure, increase the risk of developing childhood brain tumors [
14,
15]. According to the American Cancer Society (ACS), the GBM patient’s age is associated with the survival rate, which is better for young people than for old people [
16,
17,
18]. For example, the 5 year relative survival rate is 22% for patients aged 20–44 years, whereas it is 6% for patients aged 55–64 years [
17]. To diagnose glioma brain tumors accurately, different tests are required, such as neurological exams, imaging tests, and biopsy tests [
19,
20,
21]. Due to the superior soft tissue contrast of magnetic resonance imaging (MRI) scans, which allow for better visualization of the complexity and the heterogeneity of tumor regions, it is recognized as a gold imaging method to identify and localize brain tumors [
20,
22]. Glioma brain tumors have a variable prognosis and various heterogeneous histological subregions, which are reflected in their imaging phenotype [
23,
24,
25]. In general, the common brain tumor subregions are peritumoral edema, necrosis, cyst, and enhancement tumor [
26]. Different structural MRI modalities, such as T1-weighted (T1) scans, T2-weighted (T2) scans, contrast-enhanced T1-weighted (cT1) scans, and fluid-attenuated inversion recovery (FLAIR) scans, can be used to visualize different brain tumor subregions [
22]. For example, T2 and FLAIR scans visualize the peritumoral edema as a bright region [
26,
27]. Necrosis is identified as a bright region in the T2 scan but a dark region in the T1 scan, with an irregular enhancing border in the cT1 scan [
26]. The cyst is recognized as a dark, rounded region in the T1 scan and is a very bright region in the T2 scan [
26]. Another study used cT1 scans to justify the existence of the cystic component [
28]. However, the existence of a cyst is rare as GBM is commonly developed as a unilateral solid tumor [
28]. The enhancement tumor (ET) region is defined as a bright region surrounding the cystic/necrotic components, and the Multimodal Brain Tumor Segmentation challenge (BraTS) recognized it by comparing T1 and cT1 scans [
29]. The tumor core (TC), which includes both the ET region and cystic/necrotic components, can be visible in the T2 scan, while the whole tumor (WT) region is visible in the FLAIR scan [
29].
To improve the poor prognosis of GBM, various clinical investigations studied the connection among appearance, size, and location of GBM tumor subregions in relation to the poor survival rate [
30,
31,
32]. Tissue death in cancers is called necrosis, and it was found that the existence of a necrotic tumor is associated with a poor survival rate [
33]. Peritumoral edema can be defined as a characteristic feature of malignant glioma regarding the extent of neovascularization and vascular endothelial growth factor (VEGF) expression [
30,
34]. Angiogenic and vascular permeability factors associated with infiltrating tumors are identified as reasons for edema development [
35,
36], which is often presented in GBM and associated with poor prognosis [
27,
37]. Wu et al. found a shorter survival rate in glioblastoma patients due to the existence of both edema and necrosis [
26]. Qin et al. summarized that the surgical treatment of edema delayed postoperative recurrence and relapse rates [
27]. In addition, the survival rate of glioma patients is associated positively with the appearance of cystic tumor components [
32].
Due to the power of ML methods in developing accurate prediction systems that can identify complex and nonlinear patterns in different data types, they have recently been used to improve the healthcare systems in enhancing the diagnosis process and drug discovery [
38,
39], as well as in deciding a suitable treatment plan [
40,
41,
42,
43]. The term ML represents all traditional ML methods, such as the support vector machine (SVM), neural network (NN), trees, random forest (RF), and K-nearest neighbor (KNN), including deep learning (DL) methods, which are just NNs with a very deep structure. Recently, most researchers have used the term “ML” for traditional ML methods and “DL” for deep neural network methods. Various studies have involved prediction methods based on ML/DL and medical data to predict prognosis outcomes. Different types of medical images were used to develop an OST prediction system for different cancers [
44,
45,
46,
47,
48,
49]. For example, MRI images were used for glioma brain tumors and HGG tumors [
45,
48], while CT scans were used for pancreatic ductal adenocarcinoma, head and neck squamous cell carcinoma, lung, and gallbladder cancers [
44,
46,
47,
49].
To develop state-of-the-art OST prediction methods for GBM patients, the BraTS challenge provided well-processed medical data based on medical imaging information (MRI) and non-imaging information [
23,
29]. Numerous methods based on ML and radiomic features were proposed and examined [
50,
51,
52,
53,
54,
55], and details about the top ranked methods are listed in
Table 1 for BraTS 2018 and 2019. The BraTS validation and test datasets are unlabeled data as described in [
56]. The validation data were provided to evaluate developed prediction models and to choose the best model for the test phase. The test data were provided to test and evaluate the final (best) prediction model. The participant should conduct predictions and submit the results to the BraTS challenge for evaluation. From
Table 1, it is obvious that tumor size, which represents the volume information of tumor/tumor subregions, is an important radiomic feature in developing an automatic prediction model for GBM patients.
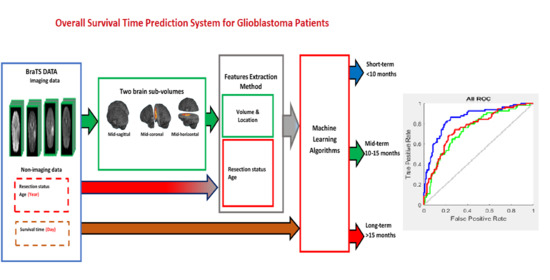
In this paper, we propose a novel method to extract volumetric and location information of GBM tumors, as well as the tumor subregions. Our proposed feature extraction method is based on calculating the volume of the GBM brain tumor and the tumor subregions in different brain function regions. To our knowledge, there is no automatic software/program that identifies each brain functional region (i.e., lobe) in structural MRI scans. Therefore, an alternative method is proposed to divide a brain volume into two sub-volumes (regions) using the brain section planes (mid-sagittal, mid-coronal, and mid-horizontal). Then, the volumes of the tumor region and subregions are calculated in each brain sub-volume. As the three brain section planes are used, three approaches are proposed to extract three different sets of radiomic features to train an ML prediction system. The BraTS 2019 dataset was used to develop our prediction system based on a classification process, which classifies a GBM patient into one of three survival groups: short-term, mid-term, and long-term.
The remainder of this paper is organized as follows:
Section 2 presents the methods and materials used to develop and evaluate the OST prediction system for GBM patients;
Section 3 lists and illustrates experiments and results that were used to test and evaluate the proposed prediction system;
Section 4 discusses the achieved results and compares them with previous studies;
Section 5 concludes this work and highlights future work to improve the performance of the proposed prediction system.
3. Results
To develop the best OST prediction model based on the proposed radiomic features and ML methods, three experiments were conducted, as described below.
In the first experiment, three sets of radiomic features (mid-sagittal, mid-coronal, and mid-horizontal were extracted from the BraTS 2019 training dataset to train an ML model using a three-class classification process. Six ML methods (NN, SVM, tree, naïve Bayes, linear discriminant, and KNN) were used to develop the OST classification model for each of the three sets of the radiomic features. Several configurations were implemented to produce the best prediction model for each ML method. The best models are listed in
Table 3,
Table 4 and
Table 5 for the mid-sagittal approach, mid-coronal approach, and mid-horizontal approach, respectively. The results show that the best performance was achieved by NN for the three sets of the radiomic features.
In the second experiment, the survival rate of GBM patients was associated with the patients’ age, which is better for young people than for old people according to the ACS [
16,
17,
18]. Thus, the age factor was used as a non-imaging feature and was combined with the radiomic features to improve the performance of the OST classification model based on NN. A simple NN architecture was used to develop the prediction system, consisting of an input layer, a hidden layer, and an output layer, as shown in
Figure 6. The size of the input layer was equal to the size of the feature vector. The hidden layer consisted of a number of nodes. The output layer consisted of three output nodes as the OST prediction system had three classification groups (short-term, mid-term, and long-term). We used the hyperbolic tangent sigmoid activation function (tanh) in the hidden layer and the softmax activation function in the classification layer. Several configurations were implemented to tune the size of the hidden layer (i.e., the number of NN nodes) for the three sets of features. The best prediction models based on the three approaches are listed in
Table 6.
For the third experiment, it was found that the surgical treatment improved the survival time for GBM patients [
27]; thus, the resection status was added to the three sets of the radiomic features, in addition to the age feature, to improve the performance of the prediction system. Then, the new feature vectors were used to train the NN classifier. Several configurations were implemented to tune the size of the hidden layer, as well as develop the best prediction rate.
Table 7 displays the best developed OST models based on feature fusions (radiomic features and non-imaging features) for the three approaches (mid-sagittal, mid-coronal, and mid-horizontal). For better understanding the performance of the developed classification models in
Table 7, confusion matrices and receiver operating characteristic curves (ROCs) are presented in
Figure 7 for the mid-sagittal, mid-coronal, and mid-horizontal approaches.
4. Discussion
The prediction system based on our radiomic feature extraction method and NN is suitable to predict prognosis outcomes for glioblastoma patients by classifying each patient into one of the three survival time groups: short-term survival (<10 months), mid-term survival (10–15 months), and long-term survival (>15 months), as shown in
Table 3,
Table 4 and
Table 5. According to the results in
Table 6 and
Table 7, the overall accuracy of the OST prediction models based on the three approaches (mid-sagittal, mid-coronal, and mid-horizontal) increased when feature fusions of radiomic and non-imaging features were used to train the NN classifier. As the survival time is better for patients after surgical treatment [
27], we expected better improvements in the system performance of Experiment 3 (
Table 7) compared to Experiment 2 (
Table 6). We believe that these slight improvements of accuracy in the validation and testing datasets of Experiment 3 were due to the unknown resection status for more than half of the samples of the data, which provided unclear descriptions for this feature. We believe that, if we have complete information about the resection status for all of the patients, the test accuracy will increase in Experiment 3.
In addition, according to the results in
Figure 7 (confusion matrices and ROCs in the test data), type II errors number of false negatives (FNs)) were more common than type I errors (number of false positive (FPs)) in the mid-term survival (Class 2), but less common than type I errors in the long-term survival (Class 3) for the three approaches of the radiomic features. Type II errors were also more common than type I errors in the short-term survival (Class 1) for both the mid-sagittal and mid-coronal radiomic feature approaches, but they were similar in number to type I errors in the mid-horizontal radiomic feature approach. Moreover, Class 2 had the worst area under the curve (AUC) compared to Class 1 and Class 3 for the three radiomic feature approaches. Thus, the accuracy of Class 2 was the worst compared to Class 1 and Class 3. There are three reasonable reasons for the reduction in accuracy of Class 2. First, the data were unbalanced, as shown in
Table 2, whereby the number of patients (i.e., samples) of Class 2 was approximately 30% lower than the number of the patients in Class 1 and Class 3. This could have affected the quality of the developed descriptors, as well as the prediction rate of Class 2. Second, the time period range of Class 2 in months is 6 months (10 months to 15 months) which is small compared to Class 1 (9 months) and Class 3 (more than 15 months, up to 60 months). This might have produced a model with descriptors that contain information from Class 1 and Class 3, which definitely affected the accuracy of Class 2. Third, the survival time of 300 days (10 months) was used to separate Class 2 and Class 1, whereas the survival time of 450 days (15 months) was used to separate Class 2 and Class 3. Thus, Class 2 has two critical zones (one with Class 1 (day 300) and another one with Class 3 (day 450), whereas Class 1 and Class 3 have only one critical zone (day 299) and (day 451) for Class 1 and Class 3, respectively. This allowed Class 2 to have samples containing features of Class 1 and Class 3. To clarify this point,
Figure 3b shows there were six patients with an overall survival time of 9 months (short-term), and two of these patients had a survival time of 296 days. Eight patients had an overall survival time of 16 months (long-term), and one of these patients had a survival time of 453 days. This means that, within more or less a few days, these patients would be considered Class 2. Furthermore, one of the patients with a survival time of 10 months (mid-term) had a survival time of 300 days, and two patients with a survival time of 15 months had a survival time of 448 days (mid-term). Thus, these patients were in a critical time interval, which makes them likely to have descriptors from other classes. We believe that increasing size of the training dataset and/or using balanced data can decrease the occurrence of type I errors and type II errors, as well as improve the performance of the prediction system.
Moreover, the test accuracy of the OST prediction model developed in this paper is better than the test accuracy of our previous work, which was based on radiomic features from eight and four brain sub-volumes and shape features [
57]. In addition, the results of our prediction system are competitive compared with the top achievements in BraTS 2018–2019 [
50,
51,
52,
53,
54,
55], as their best accuracy in the unseen dataset did not exceed 62%, as shown in
Table 1.