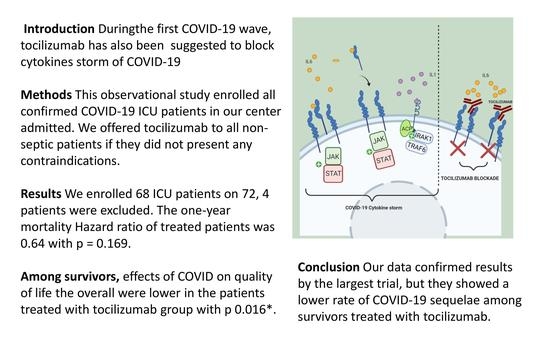
Intensive Care Patients from the First COVID-19 Wave: One-Year Survival after Tocilizumab Treatment
Abstract
:1. Introduction
2. Materials and Methods
Statistics
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Rivi, V.; Melegari, G.; Blom, J.M.C. How to humanise the COVID-19 intensive care units. BMJ Evidence-Based Med. 2021, 26, 141–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, Z.; Li, J.W.; Zhao, H.; Wang, G.Q. Cytokine release syndrome in severe COVID-19: Interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents 2020, 55, 105954. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Wang, W.; Hayek, S.S.; Chan, L.; Mathews, K.S.; Melamed, M.L.; Brenner, S.K.; Leonberg-Yoo, A.; Schenck, E.J.; Radbel, J.; et al. Association between Early Treatment with Tocilizumab and Mortality among Critically Ill Patients with COVID-19. JAMA Intern. Med. 2021, 181, 41–51. [Google Scholar] [CrossRef]
- Salvarani, C.; Dolci, G.; Massari, M.; Merlo, D.F.; Cavuto, S.; Savoldi, L.; Bruzzi, P.; Boni, F.; Braglia, L.; Turrà, C.; et al. Effect of Tocilizumab vs Standard Care on Clinical Worsening in Patients Hospitalized with COVID-19 Pneumonia: A Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Samaee, H.; Mohsenzadegan, M.; Ala, S.; Maroufi, S.S.; Moradimajd, P. Tocilizumab for treatment patients with COVID-19: Recommended medication for novel disease. Int. Immunopharmacol. 2020, 89, 107018. [Google Scholar] [CrossRef] [PubMed]
- Salama, C.; Han, J.; Yau, L.; Reiss, W.G.; Kramer, B.; Neidhart, J.D.; Criner, G.J.; Kaplan-Lewis, E.; Baden, R.; Pandit, L.; et al. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N. Engl. J. Med. 2020, 384, 20–30. [Google Scholar] [CrossRef]
- Apolone, G.; Mosconi, P. The Italian SF-36 Health Survey: Translation, validation and norming. J. Clin. Epidemiol. 1998, 51, 1025–1036. [Google Scholar] [CrossRef]
- Gamberini, L.; Mazzoli, C.A.; Sintonen, H.; Colombo, D.; Scaramuzzo, G.; Allegri, D.; Tonetti, T.; Zani, G.; Capozzi, C.; Giampalma, E.; et al. Quality of life of COVID-19 critically ill survivors after ICU discharge: 90 days follow-up. Qual. Life Res. 2021, 30, 2805–2817. [Google Scholar] [CrossRef]
- Gallo Marin, B.; Aghagoli, G.; Lavine, K.; Yang, L.; Siff, E.J.; Chiang, S.S.; Salazar-Mather, T.P.; Dumenco, L.; Savaria, M.C.; Aung, S.N.; et al. Predictors of COVID-19 severity: A literature review. Rev. Med. Virol. 2021, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Li, S.; Yu, H.; Wang, P.; Zhang, Y.; Chen, Z.; Li, Y.; Cheng, L.; Li, W.; Jia, H.; et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: A systematic review and meta-analysis. Aging 2020, 12, 12493–12503. [Google Scholar] [CrossRef] [PubMed]
- Remuzzi, A.; Remuzzi, G. COVID-19 and Italy: What next? Heal. Policy 2020, 395, 1225–1228. [Google Scholar] [CrossRef]
- Barbieri, A.; Melegari, G.; Lob, V.; Mazzali, L.; D’Amelio, L.; Giovannoni, A.; Giuliani, E. Response by Twin Italian Hub Hospitals in a Double Seismic Event: A Retrospective Observational Investigation. Prehospital Emerg. Care 2018, 22, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Senni, M. COVID-19 experience in Bergamo, Italy. Eur. Heart J. 2020, 41, 1783–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagiuoli, S.; Lorini, F.L.; Remuzzi, G. Adaptations and Lessons in the Province of Bergamo. N. Engl. J. Med. 2020, 382, e71. [Google Scholar] [CrossRef]
- Perico, L.; Tomasoni, S.; Peracchi, T.; Perna, A.; Pezzotta, A.; Remuzzi, G.; Benigni, A. COVID-19 and lombardy: TESTing the impact of the first wave of the pandemic: The prevalence of SARS-CoV-2 infection in northern Italy. EBioMedicine 2020, 61, 103069. [Google Scholar] [CrossRef]
- Melegari, G.; Giuliani, E.; Maini, G.; Barbieri, L.; Baffoni, P.; Bertellini, E.; Barbieri, A. Novel coronavirus (2019-nCov): Do you have enough intensive care units? Med. Intensiva 2020, 44, 583–585. [Google Scholar] [CrossRef]
- Grasselli, G.; Pesenti, A.; Cecconi, M. Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast during an Emergency Response. JAMA—J. Am. Med. Assoc. 2020, 323, 1545–1546. [Google Scholar] [CrossRef] [Green Version]
- Huang, E.; Jordan, S.C. Tocilizumab for Covid-19—The Ongoing Search for Effective Therapies. N. Engl. J. Med. 2020, 383, 2387–2388. [Google Scholar] [CrossRef]
- Toniati, P.; Piva, S.; Cattalini, M.; Garrafa, E.; Regola, F.; Castelli, F.; Franceschini, F.; Airò, P.; Bazzani, C.; Beindorf, E.A.; et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 2020, 19, 102568. [Google Scholar] [CrossRef]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345. [Google Scholar] [CrossRef]
- Veiga, V.C.; Prats, J.A.G.G.; Farias, D.L.C.; Rosa, R.G.; Dourado, L.K.; Zampieri, F.G.; Machado, F.R.; Lopes, R.D.; Berwanger, O.; Azevedo, L.C.P.; et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: Randomised controlled trial. BMJ 2021, 372, n84. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Fatemi, B.; Karimi Majd, Z.; Minaei, H.; Peikanpour, M.; Anjidani, N.; Taheri, A.; Dastan, F.; Mosaed, R. Efficacy and safety of Tocilizumab in severe and critical COVID-19: A Systematic Review and Meta-Analysis. Expert Rev. Clin. Immunol. 2021, 17, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, N.; Terao, K.; Mima, T.; Nakahara, H.; Takagi, N.; Kakehi, T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti–IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 2008, 112, 3959–3964. [Google Scholar] [CrossRef]
- Antwi-Amoabeng, D.; Kanji, Z.; Ford, B.; Beutler, B.D.; Riddle, M.S.; Siddiqui, F. Clinical outcomes in COVID-19 patients treated with tocilizumab: An individual patient data systematic review. J. Med. Virol. 2020, 92, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Balachandar, V.; Mahalaxmi, I.; Subramaniam, M.; Kaavya, J.; Senthil Kumar, N.; Laldinmawii, G.; Narayanasamy, A.; Janardhana Kumar Reddy, P.; Sivaprakash, P.; Kanchana, S.; et al. Follow-up studies in COVID-19 recovered patients—Is it mandatory? Sci. Total Environ. 2020, 729, 139021. [Google Scholar] [CrossRef] [PubMed]
- Chippa, V.; Aleem, A.; Anjum, F. Post Acute Coronavirus (COVID-19) Syndrome. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Taboada, M.; Moreno, E.; Cariñena, A.; Rey, T.; Pita-Romero, R.; Leal, S.; Sanduende, Y.; Rodríguez, A.; Nieto, C.; Vilas, E.; et al. Quality of life, functional status, and persistent symptoms after intensive care of COVID-19 patients. Br. J. Anaesth. 2021, 126, e110–e113. [Google Scholar] [CrossRef]
- Cortinovis, M.; Perico, N.; Remuzzi, G. Long-term follow-up of recovered patients with COVID-19. Lancet 2021, 397, 173–175. [Google Scholar] [CrossRef]
- Zanza, C.; Tassi, M.F.; Romenskaya, T.; Piccolella, F.; Abenavoli, L.; Franceschi, F.; Piccioni, A.; Ojetti, V.; Saviano, A.; Canonico, B.; et al. Lock, stock and barrel: Role of renin-angiotensin-aldosterone system in coronavirus disease 2019. Cells 2021, 10, 1752. [Google Scholar] [CrossRef]
- None, T.L.N. Long COVID: Understanding the neurological effects. Lancet Neurol. 2021, 20, 247. [Google Scholar] [CrossRef]
- Melegari, G.; Rivi, V.; Zelent, G.; Nasillo, V.; De Santis, E.; Melegari, A.; Bevilacqua, C.; Zoli, M.; Meletti, S.; Barbieri, A. Mild to severe neurological manifestations of covid-19: Cases reports. Int. J. Environ. Res. Public Health 2021, 18, 3673. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.; Nasillo, V.; Tagliafico, E.; Trenti, T.; Comoli, P.; Luppi, M. COVID-19: More than a cytokine storm. Crit. Care 2020, 24, 1–3. [Google Scholar] [CrossRef]
- Zanza, C.; Racca, F.; Longhitano, Y.; Piccioni, A.; Franceschi, F.; Artico, M.; Abenavoli, L.; Maiese, A.; Passaro, G.; Volonnino, G.; et al. Risk Management and Treatment of Coagulation Disorders Related to COVID-19 Infection. Int. J. Environ. Res. Public Health 2021, 18, 1268. [Google Scholar] [CrossRef] [PubMed]
- Fisicaro, F.; Di Napoli, M.; Liberto, A.; Fanella, M.; Di Stasio, F.; Pennisi, M.; Bella, R.; Lanza, G.; Mansueto, G. Neurological sequelae in patients with covid-19: A histopathological perspective. Int. J. Environ. Res. Public Health 2021, 18, 1415. [Google Scholar] [CrossRef] [PubMed]
- Nature Medicine. Meeting the challenge of long COVID. Nat. Med. 2020, 26, 1803. [Google Scholar] [CrossRef]
- The Lancet. The Lancet Facing up to long COVID. Lancet 2020, 396, 1861. [Google Scholar] [CrossRef]
- Del Rio, C.; Collins, L.F.; Malani, P. Long-term Health Consequences of COVID-19. JAMA—J. Am. Med. Assoc. 2020, 324, 1723–1724. [Google Scholar] [CrossRef]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef]
- Wei, Q.; Lin, H.; Wei, R.G.; Chen, N.; He, F.; Zou, D.H.; Wei, J.R. Tocilizumab treatment for COVID-19 patients: A systematic review and meta-analysis. Infect. Dis. Poverty 2021, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhang, M.; Jin, M.; Zhang, F.; Chu, Q.; Wang, X.; Chen, C.; Yue, H.; Zhang, L.; Du, R.; et al. Repurposed Tocilizumab in Patients with Severe COVID-19. J. Immunol. 2021, 206, 599–606. [Google Scholar] [CrossRef]
- Stone, J.H.; Frigault, M.J.; Serling-Boyd, N.J.; Fernandes, A.D.; Harvey, L.; Foulkes, A.S.; Horick, N.K.; Healy, B.C.; Shah, R.; Bensaci, A.M.; et al. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N. Engl. J. Med. 2020, 383, 2333–2344. [Google Scholar] [CrossRef] [PubMed]


| Tocilizumab | ||||
|---|---|---|---|---|
| Overall M ± SD or % | Treated M ± SD or % | Untreated M ± SD or % | p-Value | |
| Age (years) | 64.38 ± 10.57 | 60.19 ± 11.79 | 66.97 ± 8.93 | 0.009 * |
| Charlson Index (points) | 3.54 ± 1.57 | 3.03 ± 1.56 | 3.87 ± 1.52 | 0.036 * |
| SOFA at admission (points) | 3.67 ± 1.49 | 3.57 ± 1.57 | 3.73 ± 1.44 | 0.682 |
| SOFA at 48 h from admission (points) | 3.42 ± 2.15 | 3.24 ± 1.71 | 3.53 ± 2.41 | 0.593 |
| SAPS II at admission (points) | 32.94 ± 9.17 | 34.51 ± 9.90 | 30.46 ± 7.74 | 0.078 |
| IL 6 in the first 24 h (pg/mL) | 477.05 ± 626.91 | 462.27 ± 657.55 | 486.28 ± 615.34 | 0.882 |
| IL 6 in the first 48 h (pg/mL) | 519.16 ± 725.53 | 779.40 ± 900.80 | 287.60 ± 483.10 | 0.015 * |
| IL 6 at 7 days from admission (pg/mL) | 632.51 ± 97.91 | 1034.37 ± 1212.40 | 212.40 ± 292.71 | 0.003 * |
| Cortisonic treatment (percentage (%)) | 52.94% | 61.54% | 47.62% | 0.264 |
| Therapeutic heparin (percentage (%)) | 41.18% | 50% | 35.71% | 0.245 |
| MDR infection intra-hospital (percentage (%)) | 41.18% | 38.46 | 42.86 | 0.772 |
| One-year mortality (percentage (%)) | 48.53% | 42.31 | 52.38% | 0.576 * |
| New-onset referred symptoms among survivors (percentage (%)) | 59.38% | 35.71% | 77.78% | 0.001 * |
| Follow-Up Survivors’ Treatment | ||||
|---|---|---|---|---|
| Overall 33 (obs) | Treated (14 obs) | Untreated (19 obs) | ||
| No new-onset symptoms reported | 39.33% | 57.14% | 26.32% | |
| Referred neurological symptoms | 33.33% | 28.57% | 36.84% | |
| Referred fatigue or dyspnea | 9.09% | 0.00% | 15.79% | |
| Referred problems linked to COVID-19 hospitalization | 18.80% | 14.29% | 21.05% | p-value 0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melegari, G.; Giuliani, E.; Dallai, C.; Veronesi, L.; Bertellini, E.; Osmenaj, S.; Barbieri, A. Intensive Care Patients from the First COVID-19 Wave: One-Year Survival after Tocilizumab Treatment. J. Pers. Med. 2021, 11, 1234. https://doi.org/10.3390/jpm11111234
Melegari G, Giuliani E, Dallai C, Veronesi L, Bertellini E, Osmenaj S, Barbieri A. Intensive Care Patients from the First COVID-19 Wave: One-Year Survival after Tocilizumab Treatment. Journal of Personalized Medicine. 2021; 11(11):1234. https://doi.org/10.3390/jpm11111234
Chicago/Turabian StyleMelegari, Gabriele, Enrico Giuliani, Chiara Dallai, Lucia Veronesi, Elisabetta Bertellini, Suela Osmenaj, and Alberto Barbieri. 2021. "Intensive Care Patients from the First COVID-19 Wave: One-Year Survival after Tocilizumab Treatment" Journal of Personalized Medicine 11, no. 11: 1234. https://doi.org/10.3390/jpm11111234