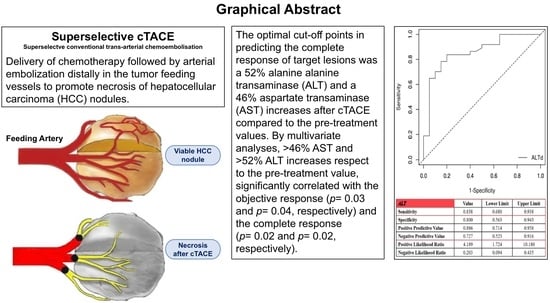
TRANS-TACE: Prognostic Role of the Transient Hypertransaminasemia after Conventional Chemoembolization for Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
- Number of nodules treated;
- Nodule location, reported as right lobe, left lobe, caudate lobe, or bilateral;
- Number of embolized segmental arteries.
- Size, measured as the maximum diameter of the lesion expressed in mm;
- Location, recorded as liver segments from 1 to 8;
- Nodule site: peripheral or central;
- Type of vascularization, recorded as intrahepatic or extrahepatic.
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Estimating the world cancer burden: Globocan 2000. Int. J. Cancer 2001, 94, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.X.; Ribes, J.; Díaz, M.; Cléries, R. Primary liver cancer: Worldwide incidence and trends. Gastroenterology 2004, 127, S5–S16. [Google Scholar] [CrossRef]
- American Cancer Society Cancer facts and figures 2015; American Cancer Society: Atlanta, GA, USA, 2015; Available online: http://www.cancer.org/acs (accessed on 6 April 2021).
- Cucchetti, A.; Trevisani, F.; Cappelli, A.; Mosconi, C.; Renzulli, M.; Pinna, A.D.; Golfieri, R. Cost-effectiveness of doxorubicin-eluting beads versus conventional trans-arterial chemo-embolization for hepatocellular carcinoma. Dig. Liver. Dis. 2016, 48, 798–805. [Google Scholar] [CrossRef]
- Liver Cancer. The Global Cancer Observatory Cancer Fact Sheets. 2018. Available online: http://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf (accessed on 8 April 2021).
- Llovet, J.M.; Beaugrand, M. Hepatocellular carcinoma: Present status and future prospects. J. Hepatol. 2003, 38, S136–S149. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [Green Version]
- Forner, A.; Real, M.I.; Varela, M.; Bruix, J. Transarterial chemoembolization for patients with hepatocellular carcinoma. Hepatol. Res. 2007, 37, S230–S237. [Google Scholar] [CrossRef]
- Okuda, K.; Ohnishi, K.; Kimura, K.; Matsutani, S.; Sumida, M.; Goto, N.; Musha, H.; Takashi, M.; Suzuki, N.; Shinagawa, T. Incidence of portal vein thrombosis in liver cirrhosis. An angiographic study in 708 patients. Gastroenterology 1985, 89, 279–286. [Google Scholar] [CrossRef]
- Facciorusso, A.; Licinio, R.; Muscatiello, N.; Di Leo, A.; Barone, M. Transarterial chemoembolization: Evidences from the literature and applications in hepatocellular carcinoma patients. World J. Hepatol. 2015, 7, 2009–2019. [Google Scholar] [CrossRef]
- Granito, A.; Marinelli, S.; Terzi, E.; Piscaglia, F.; Renzulli, M.; Venerandi, L.; Benevento, F.; Bolondi, L. Metronomic capecitabine as second-line treatment in hepatocellular carcinoma after sorafenib failure. Dig. Liver Dis. 2015, 47, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.S.; Benhamou, M.; Teyssier, Y.; Seigneurin, A.; Abousalihac, M.; Sengel, C.; Seror, O.; Ghelfi, J.; Ganne-Carrié, N.; Blaise, L.; et al. Comparison of Trans-Arterial Chemoembolization and Bland Embolization for the Treatment of Hepatocellular Carcinoma: A Propensity Score Analysis. Cancers 2021, 3, 812. [Google Scholar] [CrossRef]
- Varela, M.; Reig, M.; de la Mata, M.; Matilla, A.; Bustamante, J.; Pascual, S.; Turnes, J.; Aracil, C.; Del Val, A.; Pascasio, J.M.; et al. Treatment approach of hepatocellular carcinoma in Spain. Analysis of 705 patients from 62 centers. Med. Clin. 2010, 134, 569–576. [Google Scholar] [CrossRef]
- Giannini, E.G.; Moscatelli, A.; Pellegatta, G.; Vitale, A.; Farinati, F.; Ciccarese, F.; Piscaglia, F.; Rapaccini, G.L.; Di Marco, M.; Caturelli, E.; et al. Application of the Intermediate-Stage Subclassification to Patients With Untreated Hepatocellular Carcinoma. Am. J. Gastroenterol. 2016, 111, 70–77, Erratum in 2018, 113, 1100. [Google Scholar] [CrossRef] [PubMed]
- Raoul, J.L.; Forner, A.; Bolondi, L.; Cheung, T.T.; Kloeckner, R.; de Baere, T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use itbased on clinicalevidence. Cancer Treat. Rev. 2019, 72, 28–36. [Google Scholar] [CrossRef]
- Kishore, S.A.; Bajwa, R.; Madoff, D.C. Embolotherapeutic Strategies for Hepatocellular Carcinoma: 2020 Update. Cancers 2020, 12, 791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raoul, J.L.; Sangro, B.; Forner, A.; Mazzaferro, V.; Piscaglia, F.; Bolondi, L.; Lencioni, R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: Available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat. Rev. 2011, 37, 212–220. [Google Scholar] [CrossRef]
- The Cancer of the Liver Italian Program (CLIP) investigators. A new prognostic system for hepatocellular carcinoma: A retrospective study of 435 patients. Hepatology 1998, 28, 751–755. [Google Scholar] [CrossRef]
- The Cancer of the Liver Italian Program (CLIP) Investigators. Prospective validation of the CLIP score: A new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology 2000, 31, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Chung, H.; Osaki, Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): Its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J. Gastroenterol. 2003, 38, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Brú, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.E.; de Lope, C.R.; Bruix, J. Current strategy for staging and treatment: The BCLC update and future prospects. Semin. Liver Dis. 2010, 30, 61–74. [Google Scholar] [CrossRef]
- Cho, Y.K.; Chung, J.W.; Kim, J.K.; Ahn, Y.S.; Kim, M.Y.; Park, Y.O.; Kim, W.T.; Byun, J.H. Comparison of 7 staging systems for patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Cancer 2008, 112, 352–361. [Google Scholar] [CrossRef]
- Op den Winkel, M.; Nagel, D.; Op den Winkel, P.; Trojan, J.; Paprottka, P.M.; Steib, C.J.; Schmidt, L.; Göller, M.; Stieber, P.; Göhring, P.; et al. Transarterial chemoembolization for hepatocellular carcinoma: Development and external validation of the Munich-TACE score. Eur. J. Gastroenterol. Hepatol. 2018, 30, 44–53. [Google Scholar] [CrossRef]
- Kadalayil, L.; Benini, R.; Pallan, L.; O’Beirne, J.; Marelli, L.; Yu, D.; Hackshaw, A.; Fox, R.; Johnson, P.; Burroughs, A.K.; et al. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann. Oncol. 2013, 24, 2565–2570. [Google Scholar] [CrossRef] [PubMed]
- Hucke, F.; Pinter, M.; Graziadei, I.; Bota, S.; Vogel, W.; Müller, C.; Heinzl, H.; Waneck, F.; Trauner, M.; Peck-Radosavljevic, M.; et al. How to STATE suitability and START transarterial chemoembolization in patients with intermediate stage hepatocellular carcinoma. J. Hepatol. 2014, 61, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xia, D.; Bai, W.; Wang, E.; Sun, J.; Huang, M.; Mu, W.; Yin, G.; Li, H.; Zhao, H.; et al. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: A multicentre observational study. J. Hepatol. 2019, 70, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Child, C.G. Turcotte JG. Surgery and portal hypertension. Major Probl. Clin. Surg. 1964, 1, 1–85. [Google Scholar] [PubMed]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef]
- Wigmore, S.J.; Redhead, D.N.; Thomson, B.N.; Currie, E.J.; Parks, R.W.; Madhavan, K.K.; Garden, O.J. Postchemoembolisation syndrome--tumour necrosis or hepatocyte injury? Br. J. Cancer 2003, 89, 1423–1427. [Google Scholar] [CrossRef] [Green Version]
- Paye, F.; Farges, O.; Dahmane, M.; Vilgrain, V.; Flejou, J.F.; Belghiti, J. Cytolysis following chemoembolization for hepatocellular carcinoma. Br. J. Surg. 1999, 86, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Golfieri, R.; Renzulli, M.; Mosconi, C.; Forlani, L.; Giampalma, E.; Piscaglia, F.; Trevisani, F.; Bolondi, L.; Bologna Liver Oncology Group (BLOG). Hepatocellular carcinoma responding to superselective transarterial chemoembolization: An issue of nodule dimension? J. Vasc. Interv. Radiol. 2013, 24, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Marquez, V.; Sylvestre, M.P.; Wartelle-Bladou, C.; Bouchard, L.; Perrault, P.; Grégoire, P.; Pomier-Layrargues, G. Impact of cytolysis following transarterial chemoembolization for hepatocellular carcinoma. J. Gastrointest. Oncol. 2013, 4, 45–52. [Google Scholar] [PubMed]
- Castells, A.; Bruix, J.; Ayuso, C.; Brú, C.; Montanyà, X.; Boix, L.; Rodès, J. Transarterial embolization for hepatocellular carcinoma. Antibiotic prophylaxis and clinical meaning of postembolization fever. J. Hepatol. 1995, 22, 410–415. [Google Scholar] [CrossRef]
- Makary, M.S.; Khandpur, U.; Cloyd, J.M.; Mumtaz, K.; Dowell, J.D. Locoregional Therapy Approaches for Hepatocellular Carcinoma: Recent Advances and Management Strategies. Cancers 2020, 12, 1914. [Google Scholar] [CrossRef] [PubMed]
- Golfieri, R.; Cappelli, A.; Cucchetti, A.; Piscaglia, F.; Carpenzano, M.; Peri, E.; Ravaioli, M.; D’Errico-Grigioni, A.; Pinna, A.D.; Bolondi, L. Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (<5 cm) hepatocellular carcinomas. Hepatology 2011, 53, 1580–1589. [Google Scholar]
- Renzulli, M.; Peta, G.; Vasuri, F.; Marasco, G.; Caretti, D.; Bartalena, L.; Spinelli, D.; Giampalma, E.; D’Errico, A.; Golfieri, R. Standardization of conventional chemoembolization for hepatocellular carcinoma. Ann. Hepatol. 2021, 22, 100278. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver. Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [Green Version]



| Patients (n = 70) Median (IQR) or N. (%) | |
|---|---|
| Age (years) | 69 (61.2–77.7) |
| Gender (M/F) | 48 (68.5)/22 (31.5) |
| Milan in | 56 (80) |
| Alcohol abuse | 8 (11.4) |
| Etiology (HCV/HBV/other) | 35 (50)/6 (8.5)/29 (41.5) |
| AST (n.v. < 35 U/L) | 47 (33–80.7) |
| ALT (n.v. < 35 U/L) | 31.5 (23.25–52.7) |
| GGT (n.v. < 38 U/L) | 62.5 (35–112.5) |
| Alkaline phosphatase (n.v. 30–120 U/L) | 107.5 (80.7–144.7) |
| Total bilirubin (n.v. < 1.2 mg/dL) | 1.28 (0.79–1.64) |
| AFP (n.v. < 10 ng/mL) | 5.85 (3.4–18.8) |
| Child–Pugh Score (A/B7) | 59 (84.3)/11 (15.7) |
| Nodules per patient | 1 (1–2) |
| Number of nodules per patient: 1 2 3 | 43 (61.5) 25 (35.7) 2 (2.8) |
| Max diameter (mm) | 20 (15.25–27.25) |
| Lobe (right/left/caudate/bilobar) | 47 (67.1)/12 (17.1)/2 (2.8)/9 (13) |
| BCLC (A/B) | 31 (44.3)/39 (55.7) |
| Patients (n = 70) Median (IQR) or N. (%) | |
|---|---|
| AST (n.v. < 35 U/L) | 150.5 (55–240.8) |
| ALT (n.v. < 35 U/L) | 122 (38.2–231.7) |
| AST increase | 67% (7–488%) |
| ALT increase | 96% (7–572%) |
| GGT (n.v. < 38 U/L) | 61 (32.2–122.2) |
| Alkaline phosphatase (n.v. 30–120 U/L) | 101 (78–138) |
| Total bilirubin (n.v. < 1.2 mg/dL) | 1.52 (0.94–1.86) |
| Postembolization ascites | 7 (10) |
| Child–Pugh Score (A/B) | 53 (75.7)/17 (24.3) |
| Postembolization syndrome | 22 (31.4) |
| Univariate Analysis (OR CI 95%) | p | Multivariate Analysis (OR CI 95%) | p | |
|---|---|---|---|---|
| Age (reference ≤ 65 years) | 1.12 (0.86–2.48) | 0.16 | ||
| Gender (reference F) | 1.06 (0.78–1.3) | 0.58 | ||
| Etiology (reference HCV) | HBV: 0.77 (0.61–1.41) Other: 1.14 (0.77–3.09) | 0.3 0.13 | ||
| Child–Pugh (reference A) | 1.39 (0.88–2.11) | 0.25 | ||
| Milan in (no reference) | 0.57 (0.15–1.73) | 0.43 | ||
| AST (ref ≤ 47) | 1.004 (0.99–1.01) | 0.41 | ||
| ALT (reference ≤ 31.5) | 1.00 (0.99–1.01) | 0.87 | ||
| AFP (reference ≤ 20 UI/mL) | 1.04 (0.93–1.11) | 0.43 | ||
| GGT (reference ≤ 62.5) | 0.99 (0.45–1.47) | 0.45 | ||
| Number of treated arteries | 1.55 (0.40–3.93) | 0.44 | ||
| Max diameter (reference ≤ 20 mm) | 1.15 (1.08–1.26) | 0.001 | 1.03 (0.91–1.17) | 0.15 |
| Number of nodules (reference 1) | 0.78 (0.52–0.92) | 0.05 | 0.79 (0.65–2.51) | 0.23 |
| BCLC (reference A) | 0.48 (0.28–1.17) | 0.14 | ||
| Post-TACE ascites (no reference) | 3.11 (1.8–5.3) | <0.001 | 3.57 (0.73–5.2) | 0.35 |
| Fever (no reference) | 2.76 (0.92–7.55) | 0.15 | ||
| AST increase (reference ≤ 46%) | 1.91 (1.37–3.13) | 0.007 | 1.15 (1.10–2.89) | 0.03 |
| ALT increase (reference ≤ 52%) | 1.66 (1.28–2.42) | 0.006 | 1.40 (1.21–2.69) | 0.04 |
| Univariate Analysis (OR CI 95%) | p | Multivariate Analysis (OR CI 95%) | p | |
|---|---|---|---|---|
| Age (reference ≤ 65 years) | 1.15 (0.76–1.84) | 0.33 | ||
| Gender (reference F) | 0.98 (0.75–1.41) | 0.44 | ||
| Etiology (reference HCV) | HBV: 0.67 (0.52–1.29) Other: 1.19 (0.71–4.15) | 0.4 0.24 | ||
| Child–Pugh (reference A) | 1.18 (0.78–2.18) | 0.43 | ||
| Milan in (no reference) | 0.59 (0.11–1.13) | 0.39 | ||
| AST (reference ≤ 47 U/L) | 1.21 (0.75–1.41) | 0.11 | ||
| ALT (reference ≤ 31.5 U/L) | 1.09 (0.92–1.41) | 0.39 | ||
| AFP (reference ≤ 20 ng/mL) | 1.15 (0.69–1.71) | 0.34 | ||
| GGT (reference ≤ 62.5 U/L) | 0.89 (0.58–1.27) | 0.71 | ||
| Number of arteries | 1.39 (0.41–2.39) | 0.39 | ||
| Max diameter (reference ≤ 20 mm) | 1.02 (0.78–1.32) | 0.31 | ||
| Number of nodules (reference 1) | 0.48 (0.32–0.75) | 0.03 | 0.59 (0.45–1.51) | 0.35 |
| BCLC (reference A) | 0.65 (0.48–2.21) | 0.34 | ||
| Post-TACE ascites (no reference) | 2.38 (1.1–3.2) | 0.009 | 1.87 (0.86–2.5) | 0.15 |
| Fever (no reference) | 2.14 (0.58–4.51) | 0.09 | ||
| AST increase (reference ≤ 46%) | 2.01 (1.45–3.73) | 0.003 | 1.84 (1.17–2.98) | 0.02 |
| ALT increase (reference ≤ 52%) | 1.52 (1.19–2.22) | 0.004 | 1.42 (1.18–2.75) | 0.02 |
| Univariate Analysis (OR CI 95%) | p | Multivariate Analysis (OR CI 95%) | p | |
|---|---|---|---|---|
| Age (reference ≤ 65 years) | 1.24 (0.81–2.23) | 0.22 | ||
| Gender (reference F) | 1.12 (0.65–1.41) | 0.38 | ||
| Etiology (reference HCV) | HBV: 1.10 (0.41–2.41) Other: 1.34 (0.72–1.89) | 0.5 0.21 | ||
| Child–Pugh (reference A) | 0.98 (0.58–2.17) | 0.29 | ||
| Milan in (no reference) | 0.58 (0.22–1.43) | 0.26 | ||
| AST (reference ≤ 47 U/L) | 1.04 (0.95–1.01) | 0.41 | ||
| ALT (reference ≤ 31.5 U/L) | 1.03 (0.91–1.09) | 0.67 | ||
| AFP (reference ≤ 20 UI/mL) | 0.99 (0.95–1.21) | 0.34 | ||
| GGT (reference ≤ 62.5 U/L) | 1.12 (0.45–2.27) | 0.34 | ||
| Number of treated arteries | 1.93 (0.91–4.5) | 0.14 | ||
| Max diameter (reference ≤ 20 mm) | 1.09 (1.04–1.70) | 0.009 | 1.03 (0.88–1.53) | 0.23 |
| Number of nodules (reference 1) | 0.86 (0.52–0.91) | 0.04 | 0.84 (0.75–1.15) | 0.28 |
| BCLC (reference A) | 0.51 (0.29–0.88) | 0.03 | 0.48 (0.33–0.92) | 0.04 |
| Post-TACE ascites (no reference) | 2.52 (1.1–3.53) | 0.01 | 1.33 (0.83–4.2) | 0.28 |
| Fever (no reference) | 2.76 (0.92–7.55) | 0.15 | ||
| AST increase (reference ≤ 46%) | 1.05 (0.98–1.15) | 0.23 | ||
| ALT increase (reference ≤ 52%) | 1.05 (0.95–1.18) | 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granito, A.; Facciorusso, A.; Sacco, R.; Bartalena, L.; Mosconi, C.; Cea, U.V.; Cappelli, A.; Antonino, M.; Modestino, F.; Brandi, N.; et al. TRANS-TACE: Prognostic Role of the Transient Hypertransaminasemia after Conventional Chemoembolization for Hepatocellular Carcinoma. J. Pers. Med. 2021, 11, 1041. https://doi.org/10.3390/jpm11101041
Granito A, Facciorusso A, Sacco R, Bartalena L, Mosconi C, Cea UV, Cappelli A, Antonino M, Modestino F, Brandi N, et al. TRANS-TACE: Prognostic Role of the Transient Hypertransaminasemia after Conventional Chemoembolization for Hepatocellular Carcinoma. Journal of Personalized Medicine. 2021; 11(10):1041. https://doi.org/10.3390/jpm11101041
Chicago/Turabian StyleGranito, Alessandro, Antonio Facciorusso, Rodolfo Sacco, Laura Bartalena, Cristina Mosconi, Ugo Vittorio Cea, Alberta Cappelli, Matteo Antonino, Francesco Modestino, Nicolò Brandi, and et al. 2021. "TRANS-TACE: Prognostic Role of the Transient Hypertransaminasemia after Conventional Chemoembolization for Hepatocellular Carcinoma" Journal of Personalized Medicine 11, no. 10: 1041. https://doi.org/10.3390/jpm11101041