Is Prevention of Postoperative Vomiting Surgery Dependent? A Retrospective Cohort Study of Total Knee Arthroplasty
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kemp, W.N. Making Ether an Ideal Anaesthetic. Can. Med. Assoc. J. 1936, 34, 409–411. [Google Scholar] [PubMed]
- Minnitt, R.J. A Successful Treatment for Toxic Symptoms resulting from Ether Anaesthesia, based on a Biochemical Investigation. Proc. R. Soc. Med. 1933, 26, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Burtles, R.; Peckett, B.W. Postoperative vomiting; some factors affecting its incidence. Br. J. Anaesth. 1957, 29, 114–123. [Google Scholar] [CrossRef]
- Dolin, S.J.; Cashman, J.N.; Bland, J.M. Effectiveness of acute postoperative pain management: I. Evidence from published data. Br. J. Anaesth. 2002, 89, 409–423. [Google Scholar] [CrossRef]
- Palazzo, M.; Evans, R. Logistic regression analysis of fixed patient factors for postoperative sickness: A model for risk assessment. Br. J. Anaesth. 1993, 70, 135–140. [Google Scholar] [CrossRef]
- Apfel, C.C.; Cakmakkaya, O.S.; Frings, G.; Kranke, P.; Malhotra, A.; Stader, A.; Turan, A.; Biedler, A.; Kolodzie, K. Droperidol has comparable clinical efficacy against both nausea and vomiting. Br. J. Anaesth. 2009, 103, 359–363. [Google Scholar] [CrossRef] [Green Version]
- Apfel, C.C.; Zhang, K.; George, E.; Shi, S.; Jalota, L.; Hornuss, C.; Fero, K.E.; Heidrich, F.; Pergolizzi, J.V.; Cakmakkaya, O.S.; et al. Transdermal scopolamine for the prevention of postoperative nausea and vomiting: A systematic review and meta-analysis. Clin. Ther. 2010, 32, 1987–2002. [Google Scholar] [CrossRef]
- Gesztesi, Z.; Scuderi, P.E.; White, P.F.; Wright, W.; Wender, R.H.; D’Angelo, R.; Black, L.S.; Dalby, P.L.; MacLean, D. Substance P (Neurokinin-1) antagonist prevents postoperative vomiting after abdominal hysterectomy procedures. Anesthesiology 2000, 93, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Jokela, R.M.; Cakmakkaya, O.S.; Danzeisen, O.; Korttila, K.T.; Kranke, P.; Malhotra, A.; Paura, A.; Radke, O.C.; Sessler, D.I.; Soikkeli, A.; et al. Ondansetron has similar clinical efficacy against both nausea and vomiting. Anaesthesia 2009, 64, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Korttila, K.; Clergue, F.; Leeser, J.; Feiss, P.; Olthoff, D.; Payeur-Michel, C.; Wessel, P.; Nave, S.; Hahne, W.; Brown, R. Intravenous dolasetron and ondansetron in prevention of postoperative nausea and vomiting: A multicenter, double-blind, placebo-controlled study. Acta Anaesthesiol. Scand. 1997, 41, 914–922. [Google Scholar] [CrossRef]
- Kranke, P.; Morin, A.M.; Roewer, N.; Eberhart, L.H. Dimenhydrinate for prophylaxis of postoperative nausea and vomiting: A meta-analysis of randomized controlled trials. Acta Anaesthesiol. Scand. 2002, 46, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Wallenborn, J.; Gelbrich, G.; Bulst, D.; Behrends, K.; Wallenborn, H.; Rohrbach, A.; Krause, U.; Kuhnast, T.; Wiegel, M.; Olthoff, D. Prevention of postoperative nausea and vomiting by metoclopramide combined with dexamethasone: Randomised double blind multicentre trial. BMJ 2006, 333, 324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, A.J.; Diemunsch, P.; Lindeque, B.G.; Scheinin, H.; Helbo-Hansen, H.S.; Kroeks, M.V.; Kong, K.L. Single-dose i.v. granisetron in the prevention of postoperative nausea and vomiting. Br. J. Anaesth. 1996, 76, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Gold, B.S.; Kitz, D.S.; Lecky, J.H.; Neuhaus, J.M. Unanticipated admission to the hospital following ambulatory surgery. JAMA 1989, 262, 3008–3010. [Google Scholar] [CrossRef] [PubMed]
- Macario, A.; Weinger, M.; Carney, S.; Kim, A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth. Analg. 1999, 89, 652–658. [Google Scholar] [CrossRef]
- Gan, T.; Sloan, F.; Dear Gde, L.; El-Moalem, H.E.; Lubarsky, D.A. How much are patients willing to pay to avoid postoperative nausea and vomiting? Anesth. Analg. 2001, 92, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Raeder, J. History of postoperative nausea and vomiting. Int. Anesthesiol. Clin. 2003, 41, 1–12. [Google Scholar] [CrossRef]
- Blumfeld, J.; Cantab, M.D. The prevention of sickness after anaesthetics. Lancet 1899, 77, 833–835. [Google Scholar] [CrossRef] [Green Version]
- Davies, R.M. Some Factors affecting the Incidence of Post-anaesthetic Vomiting. Br. Med. J. 1941, 2, 578–580. [Google Scholar] [CrossRef]
- Koivuranta, M.; Laara, E.; Snare, L.; Alahuhta, S. A survey of postoperative nausea and vomiting. Anaesthesia 1997, 52, 443–449. [Google Scholar] [CrossRef]
- Apfel, C.C.; Greim, C.A.; Haubitz, I.; Goepfert, C.; Usadel, J.; Sefrin, P.; Roewer, N. A risk score to predict the probability of postoperative vomiting in adults. Acta Anaesthesiol. Scand. 1998, 42, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Apfel, C.C.; Laara, E.; Koivuranta, M.; Greim, C.A.; Roewer, N. A simplified risk score for predicting postoperative nausea and vomiting: Conclusions from cross-validations between two centers. Anesthesiology 1999, 91, 693–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinclair, D.R.; Chung, F.; Mezei, G. Can postoperative nausea and vomiting be predicted? Anesthesiology 1999, 91, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, S.; Ganeshkumar, P. Systematic Reviews and Meta-analysis: Understanding the Best Evidence in Primary Healthcare. J. Family Med. Prim. Care 2013, 2, 9–14. [Google Scholar] [PubMed]
- Sackett, D.L.; Rosenberg, W.M.; Gray, J.A.; Haynes, R.B.; Richardson, W.S. Evidence based medicine: What it is and what it isn’t. BMJ 1996, 312, 71–72. [Google Scholar] [CrossRef] [Green Version]
- Apfel, C.C.; Heidrich, F.M.; Jukar-Rao, S.; Jalota, L.; Hornuss, C.; Whelan, R.P.; Zhang, K.; Cakmakkaya, O.S. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br. J. Anaesth. 2012, 109, 742–753. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.T.M. Historical development of modern surgery in America. Adv. Hist. Stud. 2016, 5, 168–182. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.Y.; Poon, Y.Y.; Ke, T.Y.; Chiang, M.H.; Li, Y.Y.; Tsai, P.N.; Wu, S.C. Postoperative Vomiting Following Laparoscopic Cholecystectomy Is Associated with Intraoperative Fluid Administration: A Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 5305. [Google Scholar] [CrossRef]
- Poon, Y.Y.; Ke, T.Y.; Hung, K.C.; Lu, H.F.; Chiang, M.H.; Chin, J.C.; Wu, S.C. Risk Factors of Postoperative Vomiting in the Eye of “Real-World Evidence”-Modifiable and Clinical Setting-Dependent Risk Factors in Surgical Trauma Patients. J. Pers. Med. 2021, 11, 386. [Google Scholar] [CrossRef]
- Schafer, J.L. Multiple imputation: A primer. Stat. Methods Med. Res. 1999, 8, 3–15. [Google Scholar] [CrossRef]
- van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- Poon, Y.Y.; Chang, H.C.; Chiang, M.H.; Hung, K.C.; Lu, H.F.; Wang, C.H.; Chin, J.C.; Wu, S.C. “A real-world evidence” in reduction of volatile anesthetics by BIS-guided anesthesia. Sci. Rep. 2020, 10, 11245. [Google Scholar] [CrossRef] [PubMed]
- Schafer, J.L.; Schenker, N. Inference with Imputed Conditional Means. J. Am. Stat. Assoc. 2000, 95, 144–154. [Google Scholar] [CrossRef]
- Andrews, P.L. Physiology of nausea and vomiting. Br. J. Anaesth. 1992, 69 (Suppl. 1), 2S–19S. [Google Scholar] [CrossRef]
- Sanger, G.J.; Andrews, P.L. Treatment of nausea and vomiting: Gaps in our knowledge. Auton. Neurosci. 2006, 129, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Borison, H.L. The vomiting center; a critical experimental analysis. Arch. Neurol. Psychiatry 1950, 63, 928–941. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Borison, H.L. The vomiting center; its destruction by radon implantation in dog medulla oblongata. Am. J. Physiol. 1951, 166, 712–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.C.; Borison, H.L. Copper sulphate emesis; a study of afferent pathways from the gastrointestinal tract. Am. J. Physiol. 1951, 164, 520–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.C.; Borison, H.L. A new concept of organization of the central emetic mechanism: Recent studies on the sites of action of apomorphine, copper sulfate and cardiac glycosides. Gastroenterology 1952, 22, 1–12. [Google Scholar] [CrossRef]
- Apfel, C.C.; Greim, C.A.; Goepfert, C.; Grundt, D.; Usadel, J.; Sefrin, P.; Roewer, N. Postoperative vomiting. A score for prediction of vomiting risk following inhalation anesthesia. Anaesthesist 1998, 47, 732–740. [Google Scholar] [CrossRef]
- Apfel, C.C.; Greim, C.A.; Haubitz, I.; Grundt, D.; Goepfert, C.; Sefrin, P.; Roewer, N. The discriminating power of a risk score for postoperative vomiting in adults undergoing various types of surgery. Acta Anaesthesiol. Scand. 1998, 42, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.M.; Duncan, P.G.; DeBoer, D.P.; Tweed, W.A. The postoperative interview: Assessing risk factors for nausea and vomiting. Anesth. Analg. 1994, 78, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Stadler, M.; Bardiau, F.; Seidel, L.; Albert, A.; Boogaerts, J.G. Difference in risk factors for postoperative nausea and vomiting. Anesthesiology 2003, 98, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, J.E.; Moons, K.G.; Bonsel, G.J.; Kalkman, C.J. Does measurement of preoperative anxiety have added value for predicting postoperative nausea and vomiting? Anesth. Analg. 2005, 100, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Apipan, B.; Rummasak, D.; Wongsirichat, N. Postoperative nausea and vomiting after general anesthesia for oral and maxillofacial surgery. J. Dent. Anesth. Pain Med. 2016, 16, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Eberhart, L.H.; Morin, A.M.; Felbinger, T.W.; Falkner, Y.; Georgieff, M.; Seeling, W. Results of a survey of anesthetists on postoperative nausea and vomiting. Anasthesiol. Intensivmed. Notf. Schmerzther. 1998, 33, 545–551. [Google Scholar] [CrossRef]
- Kranke, P.; Apefel, C.C.; Papenfuss, T.; Rauch, S.; Lobmann, U.; Rubsam, B.; Greim, C.A.; Roewer, N. An increased body mass index is no risk factor for postoperative nausea and vomiting. A systematic review and results of original data. Acta Anaesthesiol. Scand. 2001, 45, 160–166. [Google Scholar] [CrossRef]
- Nitahara, K.; Sugi, Y.; Shono, S.; Hamada, T.; Higa, K. Risk factors for nausea and vomiting following vitrectomy in adults. Eur. J. Anaesthesiol. 2007, 24, 166–170. [Google Scholar] [CrossRef]
- Silva, A.C.; O’Ryan, F.; Poor, D.B. Postoperative nausea and vomiting (PONV) after orthognathic surgery: A retrospective study and literature review. J. Oral. Maxillofac. Surg. 2006, 64, 1385–1397. [Google Scholar] [CrossRef]
- Watcha, M.F.; White, P.F. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology 1992, 77, 162–184. [Google Scholar] [CrossRef]
- Apfel, C.C.; Kranke, P.; Katz, M.H.; Goepfert, C.; Papenfuss, T.; Rauch, S.; Heineck, R.; Greim, C.A.; Roewer, N. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: A randomized controlled trial of factorial design. Br. J. Anaesth. 2002, 88, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Eger, E.I., 2nd; Bowland, T.; Ionescu, P.; Laster, M.J.; Fang, Z.; Gong, D.; Sonner, J.; Weiskopf, R.B. Recovery and kinetic characteristics of desflurane and sevoflurane in volunteers after 8-h exposure, including kinetics of degradation products. Anesthesiology 1997, 87, 517–526. [Google Scholar] [CrossRef]
- Sneyd, J.R.; Carr, A.; Byrom, W.D.; Bilski, A.J. A meta-analysis of nausea and vomiting following maintenance of anaesthesia with propofol or inhalational agents. Eur. J. Anaesthesiol. 1998, 15, 433–445. [Google Scholar] [CrossRef]
- Anderson, B.J.; Ralph, C.J.; Stewart, A.W.; Barber, C.; Holford, N.H. The dose-effect relationship for morphine and vomiting after day-stay tonsillectomy in children. Anaesth. Intensive Care 2000, 28, 155–160. [Google Scholar] [CrossRef]
- Cann, C.; Curran, J.; Milner, T.; Ho, B. Unwanted effects of morphine-6-glucoronide and morphine. Anaesthesia 2002, 57, 1200–1203. [Google Scholar] [CrossRef]
- Langevin, S.; Lessard, M.R.; Trepanier, C.A.; Baribault, J.P. Alfentanil causes less postoperative nausea and vomiting than equipotent doses of fentanyl or sufentanil in outpatients. Anesthesiology 1999, 91, 1666–1673. [Google Scholar] [CrossRef]
- Nakajima, R.; Nakajima, Y.; Ikeda, K. Minimum alveolar concentration of sevoflurane in elderly patients. Br. J. Anaesth 1993, 70, 273–275. [Google Scholar] [CrossRef] [Green Version]
- Nickalls, R.W.; Mapleson, W.W. Age-related iso-MAC charts for isoflurane, sevoflurane and desflurane in man. Br. J. Anaesth. 2003, 91, 170–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahan, M.; Srivastava, A.; Wilson, L.; Mailis-Gagnon, A.; Midmer, D. Opioids for managing chronic non-malignant pain: Safe and effective prescribing. Can. Fam. Physician 2006, 52, 1091–1096. [Google Scholar] [PubMed]
- Kuehn, B.M. New pain guideline for older patients: Avoid NSAIDs, consider opioids. JAMA 2009, 302, 19. [Google Scholar] [CrossRef] [PubMed]
- Kaiko, R.F. Age and morphine analgesia in cancer patients with postoperative pain. Clin. Pharmacol. Ther. 1980, 28, 823–826. [Google Scholar] [CrossRef]
- Menten, J.; Desmedt, M.; Lossignol, D.; Mullie, A. Longitudinal follow-up of TTS-fentanyl use in patients with cancer-related pain: Results of a compassionate-use study with special focus on elderly patients. Curr. Med. Res. Opin. 2002, 18, 488–498. [Google Scholar] [CrossRef]
- Nasar, M.A.; McLeavy, M.A.; Knox, J. An open study of sub-lingual buprenorphine in the treatment of chronic pain in the elderly. Curr. Med. Res. Opin. 1986, 10, 251–255. [Google Scholar] [CrossRef]
- Ladha, K.S.; Wanderer, J.P.; Nanji, K.C. Age as a predictor of rescue opioid administration immediately after the emergence of general anesthesia. J. Clin. Anesth. 2015, 27, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Poon, Y.Y.; Yang, J.C.; Chou, W.Y.; Lu, H.F.; Hung, C.T.; Chin, J.C.; Wu, S.C. Is There an Optimal Timing of Adductor Canal Block for Total Knee Arthroplasty?-A Retrospective Cohort Study. J. Pers. Med. 2021, 11, 622. [Google Scholar] [CrossRef]
- De Oliveira, G.S., Jr.; Castro-Alves, L.J.; Ahmad, S.; Kendall, M.C.; McCarthy, R.J. Dexamethasone to prevent postoperative nausea and vomiting: An updated meta-analysis of randomized controlled trials. Anesth. Analg. 2013, 116, 58–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henzi, I.; Walder, B.; Tramer, M.R. Dexamethasone for the prevention of postoperative nausea and vomiting: A quantitative systematic review. Anesth. Analg. 2000, 90, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.; Wu, H.L.; Ho, S.T.; Wang, J.J. Dexamethasone prevents postoperative nausea and vomiting: Benefit versus risk. Acta Anaesthesiol. Taiwan 2011, 49, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Olaondo, L.; Carrascosa, F.; Pueyo, F.J.; Monedero, P.; Busto, N.; Saez, A. Combination of ondansetron and dexamethasone in the prophylaxis of postoperative nausea and vomiting. Br. J. Anaesth. 1996, 76, 835–840. [Google Scholar] [CrossRef]

| Features | N (%)/Median (IQR) (N = 665) | None-POV (N = 578) | POV (N = 87) | p Value |
|---|---|---|---|---|
| Sex | ||||
| Male | 178 (26.8%) | 167 (28.9%) | 11 (12.6%) | 0.001 |
| Female | 487 (73.2%) | 411 (71.1%) | 76 (87.4%) | |
| Age (years) | 70 (65–75) | 70 (65–75) | 71 (65–76) | 0.731 |
| Weight (kg) | 67 (59–76) | 68 (60–77) | 62 (55–70) | <0.001 |
| ACB Block | ||||
| None | 287 (43.2%) | 243 (42.0%) | 44 (50.6%) | 0.167 |
| Pre-operative | 226 (34.0%) | 204 (35.3%) | 22 (25.3%) | |
| Post-operative | 152 (22.9%) | 131 (22.7%) | 21 (24.1%) | |
| ASA | ||||
| II | 408 (61.4%) | 351 (60.7%) | 57 (65.5%) | 0.422 |
| III | 257 (38.6%) | 227 (39.3%) | 30 (34.5%) | |
| Anesthesia time (h) | 3.17 (2.83–3.50) | 3.18 (2.85–3.50) | 3.07 (2.75–3.45) | 0.071 |
| Apfel Score | ||||
| 0 | 56 (8.4%) | 51 (8.8%) | 5 (5.7%) | 0.570 |
| 1 | 154 (23.2%) | 137 (23.7%) | 17 (19.5%) | |
| 2 | 311 (46.8%) | 267 (46.2%) | 44 (50.6%) | |
| ≥3 | 144 (21.7%) | 123 (21.3%) | 21 (24.1%) | |
| Charlson Comorbidity Index | ||||
| 0 | 173 (26.0%) | 152 (26.3%) | 21 (24.1%) | 0.410 |
| 1 | 282 (42.4%) | 247 (42.7%) | 35 (40.2%) | |
| 2 | 138 (20.8%) | 121 (20.9%) | 17 (19.5%) | |
| ≥3 | 72 (10.8%) | 58 (10.0%) | 14 (16.1%) |
| Variables (Unit) | N (%)/ | Univariate | Multivariable | ||
|---|---|---|---|---|---|
| Median (IQR) | OR (95% CI) | p-Value | OR (95% CI) | p Value | |
| Sex-Male | 178 (26.8%) | 1 | 1 | ||
| Sex-Female | 487 (73.2%) | 2.81 (1.46–5.42) | 0.002 | 2.49 (1.19–5.20) | 0.015 |
| Age- | 70 (65–75) | 1.01 (0.98–1.04) | 0.706 | 1.00 (0.97–1.03) | 0.977 |
| ACB Block-None | 287 (43.2%) | 1 | 1 | ||
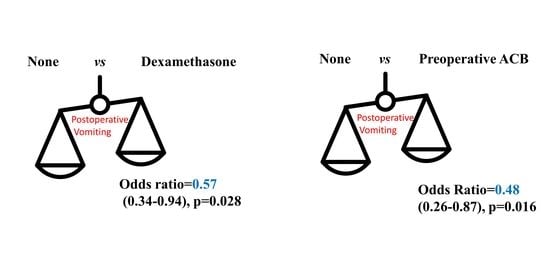
| Nerve Block-Pre-op | 226 (34.0%) | 0.60 (0.35–1.03) | 0.062 | 0.48 (0.26–0.87) | 0.016 |
| Nerve Block-Post-op | 152 (22.9%) | 0.89 (0.51–1.55) | 0.671 | 0.66 (0.36–1.22) | 0.188 |
| Weight (kg) | 67 (59–76) | 0.97 (0.95–0.99) | 0.001 | 0.98 (0.95–1.00) | 0.065 |
| ASA II | 408 (61.4%) | 1 | 1 | ||
| ASA III | 257 (38.6%) | 0.81 (0.51–1.31) | 0.393 | 0.74 (0.44–1.24) | 0.252 |
| Apfel Score 0 | 56 (8.4%) | 1 | 1 | ||
| Apfel Score 1 | 154 (23.2%) | 1.27 (0.44–3.61) | 0.659 | 1.72 (0.56–5.27) | 0.344 |
| Apfel Score 2 | 311 (46.8%) | 1.68 (0.64–4.44) | 0.295 | 1.68 (0.58–4.80) | 0.337 |
| Apfel Score 3&4 | 144 (21.7%) | 1.74 (0.62–4.87) | 0.290 | 2.19 (0.70–6.85) | 0.180 |
| Duration (h) | 3.17 (2.83–3.50) | 0.69 (0.45–1.05) | 0.081 | 0.70 (0.44–1.11) | 0.133 |
| Sevoflurane consumption (mL/h) | 0.20 (0.17–0.25) | 55.04 (1.50–2019.69) | 0.029 | 2.84 (0.04–201.49) | 0.631 |
| Crystalloid (mL/h/kg) | 2.51 (2.04–3.28) | 0.96 (0.76–1.21) | 0.734 | 0.82 (0.61–1.11) | 0.199 |
| Intraoperative Urine (mL/h/kg) | 1.00 (0.00–2.02) | 0.89 (0.74–1.08) | 0.224 | 0.87 (0.70–1.07) | 0.172 |
| PCA-none | 616 (92.6%) | 1 | 1 | ||
| PCA-Yes | 49 (7.4%) | 0.57 (0.20–1.63) | 0.295 | 0.73 (0.21–2.57) | 0.624 |
| Kinds of anti-emetics-none | 296 (44.5%) | 1 | 1 | ||
| One | 333 (50.1%) | 0.66 (0.42–1.05) | 0.081 | 0.57 (0.34–0.94) | 0.028 |
| Two | 36 (5.4%) | 0.48 (0.14–1.64) | 0.241 | 0.40 (0.09–1.75) | 0.224 |
| Kinds of anti-hypertension-none | 223 (33.5%) | 1 | 1 | ||
| One | 349 (52.5%) | 1.00 (0.61–1.64) | 0.996 | 0.94 (0.55–1.61) | 0.823 |
| Two | 93 (14.0%) | 0.78 (0.36–1.66) | 0.511 | 0.72 (0.32–1.63) | 0.424 |
| Intraoperative MME (mg/kg) | 0.23 (0.18–0.29) | 10.83 (1.51–77.69) | 0.018 | 7.04 (0.73–68.03) | 0.092 |
| MME at PACU (mg/kg) | 0.02 (0.01–0.02) | 0.62 (0.00–1157.62) | 0.901 | 2.03 (0.00–5332.59) | 0.860 |
| MME at Ward (mg/kg) | 0.10 (0.09–0.11) | 0.44 (0.07–2.75) | 0.377 | 0.59 (0.08–4.38) | 0.607 |
| Variables (Unit) | N (%)/ | Multivariable | |
|---|---|---|---|
| Median (IQR) | OR (95% CI) | p Value | |
| Sex-Male | 178 (26.8%) | 1 | |
| Sex-Female | 487 (73.2%) | 2.58(1.29–5.14) | 0.007 |
| ACB Block-None | 287 (43.2%) | 1 | |
| Nerve Block-Pre-op | 226 (34.0%) | 0.53(0.30–0.93) | 0.028 |
| Nerve Block-Post-op | 152 (22.9%) | 0.71(0.39–1.28) | 0.251 |
| Weight (kg) | 67 (59–76) | 0.98(0.96–1.00) | 0.046 |
| Duration (h) | 3.17 (2.83–3.50) | 0.70(0.45–1.09) | 0.110 |
| Intraoperative Urine (mL/h/kg) | 1.00 (0.00–2.02) | 0.83(0.68–1.01) | 0.059 |
| Kinds of anti-emetics-none | 296 (44.5%) | 1 | |
| One | 333 (50.1%) | 0.62(0.38–1.01) | 0.054 |
| Two | 36 (5.4%) | 0.38(0.11–1.35) | 0.134 |
| Intraoperative MME (mg/kg) | 0.23 (0.18–0.29) | 6.28(0.89–44.53) | 0.066 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poon, Y.-Y.; Hung, K.-C.; Chou, W.-Y.; Wang, C.-H.; Hung, C.-T.; Chin, J.-C.; Wu, S.-C. Is Prevention of Postoperative Vomiting Surgery Dependent? A Retrospective Cohort Study of Total Knee Arthroplasty. J. Pers. Med. 2021, 11, 1018. https://doi.org/10.3390/jpm11101018
Poon Y-Y, Hung K-C, Chou W-Y, Wang C-H, Hung C-T, Chin J-C, Wu S-C. Is Prevention of Postoperative Vomiting Surgery Dependent? A Retrospective Cohort Study of Total Knee Arthroplasty. Journal of Personalized Medicine. 2021; 11(10):1018. https://doi.org/10.3390/jpm11101018
Chicago/Turabian StylePoon, Yan-Yuen, Kuo-Chuan Hung, Wen-Yi Chou, Chih-Hsien Wang, Chao-Ting Hung, Jo-Chi Chin, and Shao-Chun Wu. 2021. "Is Prevention of Postoperative Vomiting Surgery Dependent? A Retrospective Cohort Study of Total Knee Arthroplasty" Journal of Personalized Medicine 11, no. 10: 1018. https://doi.org/10.3390/jpm11101018