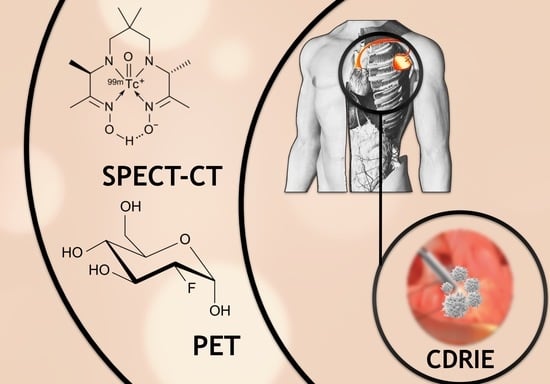
The Diagnostic Value of 99mTc-HMPAO-Labelled White Blood Cell Scintigraphy and 18F-FDG PET/CT in Cardiac Device-Related Infective Endocarditis—A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Direct Comparison of 99mTc-HMPAO-SPECT/CT and 18F-FDG PET/CT
3.2. 99mTc-HMPAO-SPECT/CT
3.3. 18F-FDG PET/CT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kirkfeldt, R.E.; Johansen, J.B.; Nohr, E.A.; Jørgensen, O.D.; Nielsen, J.C. Complications after cardiac implantable electronic device implantations: An analysis of a complete, nationwide cohort in Denmark. Eur. Heart J. 2013, 35, 1186–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.-P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis. Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef] [PubMed]
- Greenspon, A.J.; Patel, J.D.; Lau, E.; Ochoa, J.; Frisch, D.R.; Ho, R.T.; Pavri, B.B.; Kurtz, S.M. 16-Year Trends in the Infection Burden for Pacemakers and Implantable Cardioverter-Defibrillators in the United States: 1993 to 2008. J. Am. Coll. Cardiol. 2011, 58, 1001–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongiorni, M.G.; Kennergren, C.; Butter, C.; Deharo, J.C.; Kutarski, A.; Rinaldi, C.; Romano, S.L.; Maggioni, A.P.; Andarala, M.; Auricchio, A.; et al. The European Lead Extraction ConTRolled (ELECTRa) study: A European Heart Rhythm Association (EHRA) Registry of Transvenous Lead Extraction Outcomes. Eur. Heart J. 2017, 38, 2995–3005. [Google Scholar] [CrossRef] [PubMed]
- Kaura, A.; Dworakowska, D.; Dworakowski, R. Infective endocarditis—Cinderella in cardiology. Kardiologia Polska 2017, 75, 965–974. [Google Scholar] [CrossRef] [Green Version]
- Erba, P.A.; Lancellotti, P.; Vilacosta, I.; Gaemperli, O.; Rouzet, F.; Hacker, M.; Signore, A.; Slart, R.H.J.A.; Habib, G. Recommendations on nuclear and multimodality imaging in IE and CIED infections. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1795–1815. [Google Scholar] [CrossRef]
- Juneau, D.; Golfam, M.; Hazra, S.; Erthal, F.; Zuckier, L.; Bernick, J.; Wells, G.A.; Beanlands, R.S.; Chow, B.J. Molecular Imaging for the diagnosis of infective endocarditis: A systematic literature review and meta-analysis. Int. J. Cardiol. 2018, 253, 183–188. [Google Scholar] [CrossRef]
- Mahmood, M.; Abu Saleh, O. The Role of 18-F FDG PET/CT in Imaging of Endocarditis and Cardiac Device Infections. Semin. Nucl. Med. 2020, 50, 319–330. [Google Scholar] [CrossRef]
- Kubota, R.; Yamada, S.; Kubota, K.; Ishiwata, K.; Tamahashi, N.; Ido, T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: High accumulation in macrophages and granulation tissues studied by microautoradiography. J. Nucl. Med. 1992, 33. [Google Scholar]
- Ishimori, T.; Saga, T.; Mamede, M.; Kobayashi, H.; Higashi, T.; Nakamoto, Y.; Sato, N.; Konishi, J. Increased (18)F-FDG uptake in a model of inflammation: Concanavalin A-mediated lymphocyte activation. J. Nucl. Med. 2002, 43, 658–663. [Google Scholar] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Calais, J.; Touati, A.; Grall, N.; Laouénan, C.; Benali, K.; Mahida, B.; Vigne, J.; Hyafil, F.; Iung, B.; Duval, X.; et al. Diagnostic Impact of 18 F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography and White Blood Cell SPECT/Computed Tomography in Patients With Suspected Cardiac Implantable Electronic Device Chronic Infection. Circ. Cardiovasc. Imaging 2019, 12, e007188. [Google Scholar] [CrossRef]
- Erba, P.A.; Sollini, M.; Conti, U.; Bandera, F.; Tascini, C.; De Tommasi, S.M.; Zucchelli, G.; Doria, R.; Menichetti, F.; Bongiorni, M.G.; et al. Radiolabeled WBC Scintigraphy in the Diagnostic Workup of Patients With Suspected Device-Related Infections. JACC Cardiovasc. Imaging 2013, 6, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Małecka, B.A.; Ząbek, A.; Dębski, M.; Szot, W.; Holcman, K.; Boczar, K.; Ulman, M.; Lelakowski, J.; Kostkiewicz, M. The usefulness of SPECT-CT with radioisotope-labeled leukocytes in diagnosing lead-dependent infective endocarditis. Adv. Clin. Exp. Med. 2018, 28, 113–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holcman, K.; Małecka, B.; Rubiś, P.; Ząbek, A.; Szot, W.; Boczar, K.; Leśniak-Sobelga, A.; Hlawaty, M.; Wiśniowska-Śmiałek, S.; Stępień, A.; et al. The role of 99mTc-HMPAO-labelled white blood cell scintigraphy in the diagnosis of cardiac device-related infective endocarditis. Eur. Heart J. Cardiovasc. Imaging 2019, 21, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Holcman, K.; Rubiś, P.; Ząbek, A.; Ćmiel, B.; Szot, W.; Boczar, K.; Wiśniowska-Śmiałek, S.; Stępień, A.; Małecka, B.; Podolec, P.; et al. The Prognostic Value of 99mTc-HMPAO-Labeled Leucocyte SPECT/CT in Cardiac Device-Related Infective Endocarditis. JACC Cardiovasc. Imaging 2020, 13, 1739–1751. [Google Scholar] [CrossRef] [PubMed]
- Salomäki, S.P.; Saraste, A.; Kemppainen, J.; Hurme, S.; Knuuti, J.; Nuutila, P.; Seppänen, M.; Roivainen, A.; Airaksinen, J.; Salo, T.; et al. 18F-FDG positron emission tomography/computed tomography of cardiac implantable electronic device infections. J. Nucl. Cardiol. 2020, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Alfonso, B.; Casanovas, M.M.; Urda, V.C.; Marcos, M.C.; Romero, I.S.; Ramos-Martínez, A. PET/CT with 18F-FDG in suspected intracardiac device-related infections: Analysis of performance and diagnostic usefulness. Rev. Esp. Cardiol. 2020, 74, 238–246. [Google Scholar] [CrossRef]
- Cautela, J.; Alessandrini, S.; Cammilleri, S.; Giorgi, R.; Richet, H.; Casalta, J.-P.; Habib, G.; Raoult, D.; Mundler, O.; Deharo, J.-C. Diagnostic yield of FDG positron-emission tomography/computed tomography in patients with CEID infection: A pilot study. Europace 2012, 15, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Rubini, G.; Ferrari, C.; Carretta, D.; Santacroce, L.; Ruta, R.; Iuele, F.; Lavelli, V.; Merenda, N.; D’Agostino, C.; Sardaro, A.; et al. Usefulness of 18F-FDG PET/CT in Patients with Cardiac Implantable Electronic Device Suspected of Late Infection. J. Clin. Med. 2020, 9, 2246. [Google Scholar] [CrossRef] [PubMed]
- Jerónimo, A.; Olmos, C.; Vilacosta, I.; Ortega-Candil, A.; Rodríguez-Rey, C.; Pérez-Castejón, M.J.; Fernández-Pérez, C.; Pérez-García, C.N.; García-Arribas, D.; Ferrera, C.; et al. Accuracy of 18F-FDG PET/CT in patients with the suspicion of cardiac implantable electronic device infections. J. Nucl. Cardiol. 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Leccisotti, L.; Perna, F.; Lago, M.; Leo, M.; Stefanelli, A.; Calcagni, M.L.; Pelargonio, G.; Narducci, M.L.; Bencardino, G.; Bellocci, F.; et al. Cardiovascular implantable electronic device infection: Delayed vs standard FDG PET-CT imaging. J. Nucl. Cardiol. 2014, 21, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, J.-F.; Philippon, F.; Tessier, M.; Guimond, J.; Molin, F.; Champagne, J.; Nault, I.; Blier, L.; Nadeau, M.; Charbonneau, L.; et al. Usefulness of Fluorine-18 Positron Emission Tomography/Computed Tomography for Identification of Cardiovascular Implantable Electronic Device Infections. J. Am. Coll. Cardiol. 2012, 59, 1616–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensimhon, L.; Lavergne, T.; Hugonnet, F.; Mainardi, J.-L.; Latremouille, C.; Maunoury, C.; Lepillier, A.; Le Heuzey, J.-Y.; Faraggi, M. Whole body [18F]fluorodeoxyglucose positron emission tomography imaging for the diagnosis of pacemaker or implantable cardioverter defibrillator infection: A preliminary prospective study. Clin. Microbiol. Infect. 2011, 17, 836–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diemberger, I.; Bonfiglioli, R.; Martignani, C.; Graziosi, M.; Biffi, M.; Lorenzetti, S.; Ziacchi, M.; Nanni, C.; Fanti, S.; Boriani, G. Contribution of PET imaging to mortality risk stratification in candidates to lead extraction for pacemaker or defibrillator infection: A prospective single center study. Eur. J. Nucl. Med. Mol. Imaging 2018, 46, 194–205. [Google Scholar] [CrossRef]
- Tlili, G.; Amroui, S.; Mesguich, C.; Riviere, A.; Bordachar, P.; Hindié, E.; Bordenave, L. High performances of 18F-fluorodeoxyglucose PET-CT in cardiac implantable device infections: A study of 40 patients. J. Nucl. Cardiol. 2015, 22, 787–798. [Google Scholar] [CrossRef]
- Graziosi, M.; Nanni, C.; Lorenzini, M.; Diemberger, I.; Bonfiglioli, R.; Pasquale, F.; Ziacchi, M.; Biffi, M.; Martignani, C.; Bartoletti, M.; et al. Role of 18F-FDG PET/CT in the diagnosis of infective endocarditis in patients with an implanted cardiac device: A prospective study. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1617–1623. [Google Scholar] [CrossRef]
- Ploux, S.; Riviere, A.; Amraoui, S.; Whinnett, Z.; Barandon, L.; Lafitte, S.; Ritter, P.; Papaioannou, G.; Clementy, J.; Jais, P.; et al. Positron emission tomography in patients with suspected pacing system infections may play a critical role in difficult cases. Heart Rhythm. 2011, 8, 1478–1481. [Google Scholar] [CrossRef]
- Ahmed, F.Z.; James, J.; Cunnington, C.; Motwani, M.; Fullwood, C.; Hooper, J.; Burns, P.; Qamruddin, A.; Al-Bahrani, G.; Armstrong, I.; et al. Early diagnosis of cardiac implantable electronic device generator pocket infection using ¹⁸F-FDG-PET/CT. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 521–530. [Google Scholar] [CrossRef] [Green Version]
- Schünemann, H.J.; Oxman, A.D.; Brozek, J.; Glasziou, P.; Jaeschke, R.; Vist, G.E.; Williams, J.W., Jr.; Kunz, R.; Craig, J.; Montori, V.; et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008, 336, 1106–1110. [Google Scholar] [CrossRef] [Green Version]
- Habib, G.; Lancellotti, P.; Erba, P.-A.; Sadeghpour, A.; Meshaal, M.; Sambola, A.; Furnaz, S.; Citro, R.; Ternacle, J.; Donal, E.; et al. The ESC-EORP EURO-ENDO (European Infective Endocarditis) registry. Eur. Heart J. Qual. Care Clin. Outcomes 2019, 5, 202–207, Erratum in Eur. Heart J. Qual. Care Clin. Outcomes 2020, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Miro, J.M.; Ambrosioni, J. Infective endocarditis: An ongoing global challenge. Eur. Heart J. 2019, 40, 3233–3236. [Google Scholar] [CrossRef] [PubMed]
- Migaj, J.; Oko-Sarnowska, Z.; Kałużna-Oleksy, M.; Katarzyńska-Szymańska, A.; Lesiak, M.; Mitkowski, P. Atypical presentation of cardiac device related infectious endocarditis and complicated follow-up. Pol. Arch. Intern. Med. 2018, 128, 780–782. [Google Scholar] [CrossRef]
- Sohail, M.R.; Henrikson, C.A.; Braid-Forbes, M.J.; Forbes, K.; Lerner, D.J. Increased Long-Term Mortality in Patients with Cardiovascular Implantable Electronic Device Infections. Pacing Clin. Electrophysiol. 2014, 38, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Viganego, F.; O'Donoghue, S.; Eldadah, Z.; Shah, M.H.; Rastogi, M.; Mazel, J.A.; Platia, E.V. Effect of Early Diagnosis and Treatment With Percutaneous Lead Extraction on Survival in Patients With Cardiac Device Infections. Am. J. Cardiol. 2012, 109, 1466–1471. [Google Scholar] [CrossRef]
- Blomström-Lundqvist, C.; Traykov, V.; Erba, P.A.; Burri, H.; Nielsen, J.C.; Bongiorni, M.G.; Poole, J.; Boriani, G.; Costa, R.; Deharo, J.-C.; et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections—endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Europace 2019, 22, 515–549. [Google Scholar] [CrossRef]
- Kawada, K.; Iwamoto, M.; Sakai, Y. Mechanisms underlying18F-fluorodeoxyglucose accumulation in colorectal cancer. World J. Radiol. 2016, 8, 880–886. [Google Scholar] [CrossRef]
- Memmott, M.J.; James, J.; Armstrong, I.S.; Tout, D.; Ahmed, F. The performance of quantitation methods in the evaluation of cardiac implantable electronic device (CIED) infection: A technical review. J. Nucl. Cardiol. 2015, 23, 1457–1466. [Google Scholar] [CrossRef]
- Pijl, J.P.; Kwee, T.C.; Slart, R.H.J.A.; Glaudemans, A.W.J.M. PET/CT Imaging for Personalized Management of Infectious Diseases. J. Pers. Med. 2021, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Signore, A.; Jamar, F.; Israel, O.; Buscombe, J.; Martin-Comin, J.; Lazzeri, E. Clinical indications, image acquisition and data interpretation for white blood cells and anti-granulocyte monoclonal antibody scintigraphy: An EANM procedural guideline. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1816–1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Vries, E.F.; Roca, M.; Jamar, F.; Israel, O.; Signore, A. Guidelines for the labelling of leucocytes with (99m)Tc-HMPAO. Inflammation/Infection Taskgroup of the European Association of Nuclear Medicine. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 842–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Signore, A.; Glaudemans, A.W.J.M.; Malviya, G.; Lazzeri, E.; Prandini, N.; Viglietti, A.L.; De Vries, E.F.J.; Dierckx, R.A.J.O. Development and testing of a new disposable sterile device for labelling white blood cells. Q. J. Nucl. Med. Mol. Imaging 2012, 56, 400–408. [Google Scholar] [PubMed]
- Holcman, K.; Szot, W.; Rubiś, P.; Leśniak-Sobelga, A.; Hlawaty, M.; Wiśniowska-Śmiałek, S.; Małecka, B.; Ząbek, A.; Boczar, K.; Stępień, A.; et al. 99mTc-HMPAO-labeled leukocyte SPECT/CT and transthoracic echocardiography diagnostic value in infective endocarditis. Int. J. Cardiovasc. Imaging 2018, 35, 749–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husmann, L.; Cohort, A.T.V.; Huellner, M.W.; Ledergerber, B.; Anagnostopoulos, A.; Stolzmann, P.; Sah, B.-R.; Burger, I.A.; Rancic, Z.; Hasse, B. Comparing diagnostic accuracy of 18F-FDG-PET/CT, contrast enhanced CT and combined imaging in patients with suspected vascular graft infections. Eur. J. Nucl. Med. Mol. Imaging 2018, 46, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Sah, B.-R.; Husmann, L.; Mayer, D.; Scherrer, A.; Rancic, Z.; Puippe, G.; Weber, R.; Hasse, B. Diagnostic Performance of 18F-FDG-PET/CT in Vascular Graft Infections. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Dilsizian, V. Molecular Imaging of Cardiovascular Device Infection: Targeting the Bacteria or the Host–Pathogen Immune Response? J. Nucl. Med. 2020, 61, 319–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slart, R.H.J.A.; Glaudemans, A.W.J.M.; Gheysens, O.; Lubberink, M.; Kero, T.; Dweck, M.R.; Habib, G.; Gaemperli, O.; Saraste, A.; Gimelli, A.; et al. Procedural recommendations of cardiac PET/CT imaging: Standardization in inflammatory-, infective-, infiltrative-, and innervation (4Is)-related cardiovascular diseases: A joint collaboration of the EACVI and the EANM. Eur. J. Nucl. Med. Mol. Imaging 2020, 48, 1016–1039. [Google Scholar] [CrossRef]
- Husmann, L.; Ledergerber, B.; Anagnostopoulos, A.; Stolzmann, P.; Sah, B.R.; Burger, I.A.; Pop, R.; Weber, A.; Mayer, D.; Rancic, Z.; et al. The role of FDG PET/CT in therapy control of aortic graft infection. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1987–1997. [Google Scholar] [CrossRef]

| Study | Inclusion | Number of Cases and Controls | Gold Standard | Diagnostic Accuracy 1 | Quality 2 |
|---|---|---|---|---|---|
| 18F-FDG PET/CT (n = 737, 539 cases and 198 controls) | |||||
| Rodríguez-Alfonso, B. | retrospective | 44 | expert multidisciplinary team | sensitivity 84.0% (65.3–93.6%), specificity 94.7% (75.4–99.1%), PPV 95.5% (78.2–99.2%), NPV 81.8% (65.7–97.9%); LDI: sensitivity 84.2% (62.4–94.5%), specificity 96% (80.5–99.3%), PPV 94.1% (73–99%), NPV 88.9% (71.9–96.1%) | low |
| Ahmed, F.Z. | prospective | 46 and 40 controls | lead cultures, clinical assessment by an experienced cardiologist | group which underwent extraction (n = 32): sensitivity 97% (84–100%), specificity 98% (90–100%); group treated conservatively (n = 14) sensitivity 76% (61–87%), specificity 100% (87–100%) | high |
| Ploux, S. | prospective | 10 and 40 controls | lead cultures and 12-month follow up | sensitivity 100%, specificity 93%, PPV 66%, NPV 100% | low |
| Graziosi, M. | prospective | 27 | expert multidisciplinary team, modified Duke criteria, 6-month follow-up | sensitivity 63%, specificity 86%, PPV 77%, NPV 76% | low |
| Tlili, G. | retrospective | 40 and 40 controls | clinical evaluation, lead cultures and 12-month follow up | accuracy 90%, sensitivity 83%, specificity 95%, PPV 94%, NPV 88% | low |
| Diemberger, I. | prospective | 105 | expert multidisciplinary team, modified Duke criteria, 6–12-month follow-up | sensitivity 91.4% ‘Cold Closed Pocket’ associated with mortality: HR 2.84 (1.37–5.89; p = 0.005) | high |
| Bensimhon, L. | prospective | 21 and 14 controls | lead cultures, modified Duke criteria, 6-month follow-up | accuracy 90.4%, sensitivity 80%, specificity 100%, PPV 100%, NPV 84.6% | low |
| Sarrazin, J.F. | prospective | 42 and 24 controls | Duke criteria, conventional tests | sensitivity 88.6% (72.3–96.3%), specificity 85.7% (42–99.2%) | high |
| Leccisotti, L. | prospective | 27 and 15 controls | bacteriological analysis, follow-up (at least 3 months) for control group | accuracy 93% (76–99%), sensitivity 86% (65–97%), specificity 100% (48–100%) delayed 18F-FDG PET/CT: accuracy 91% (71–99%), sensitivity 91% (71–99%), specificity 100% (48–100%) | high |
| Jerónimo, A. | prospective | 63 | clinical data, lead cultures, TEE, pulmonary embolisms detected by PET/CT | sensitivity 38.5% (11.7–64.5%), specificity 98% (89.5–99.6%), PPV 83.3% (43.6–97%), NPV 86% (74.7–92.7%); LDI: sensitivity 72.2% (49.1–87.5%), specificity 95.6% (85.2–98.8%), PPV 86.7% (62.1–96.3%), NPV 89.6% (77.8–94.5%) | high |
| Calais, J. | retrospective | 48 | multidisciplinary expert team, lead cultures, Duke-Li criteria, at least 3-month follow-up | sensitivity 80% (51.9–95.7%), specificity 90.9% (75.7–98.1%), PPV 80% (56.9–92.4%), NPV 90.9%, (78.3–96.5%); diagnostic criteria including 18F-FDG PET/CT: sensitivity 100.0% (78.2–100%), specificity 84.9% (68.1–94.9%), PPV 75.0% (57.2–87.1%), NPV 100% | high |
| Salomäki, S.P. | prospective | 30 and 10 controls | modified Duke Criteria, follow-up | sensitivity 90%, specificity 73%, PPV 75%, NPV 89% | low |
| Cautela, J. | prospective | 21 | lead cultures, Duke criteria | sensitivity 30.8% (9.1–61.4%), specificity 62.5% (24.5–91.5%); LDI: sensitivity 86.7% (59.5–98.3%), specificity 100% (42.1–100%) | low |
| Rubini, G. | retrospective | 15 and 15 controls | clinical guidelines, lead cultures | accuracy 86.67% (59.54–98.34%), sensitivity 90.91% (58.72–99.77%), specificity 75% (19.41–99.37%), PPV 90.91% (64.45–98.22%), NPV 75% (29.86–95.48%) | low |
| 99mTc-HMPAO-SPECT/CT (n = 357) | |||||
| Calais, J. | retrospective | 48 | multidisciplinary expert team, lead cultures, Duke-Li criteria, at least 3-month follow-up | sensitivity 60% (32.3–83.7%), specificity 100% (89.4–100%), PPV 100%, NPV 84.6% (74.7–91.1%) diagnostic criteria including 99mTc-HMPAO-SPECT/CT: sensitivity 93.3% (68.1–99.8%), specificity 90.9% (75.7–98.1%), PPV 82.4% (61.1–93.3%), NPV 96.8% (81.8–99.5%) | high |
| Małecka, B. | prospective | 40 | modified Duke criteria | sensitivity 73.7% (55.1–86.1%), specificity 81% (64.2–92.2%), PPV 77.8% (58.2–90.9%), NPV 77.3% (61.3–88%) | low |
| Holcman, K. | prospective | 103 | multidisciplinary expert team, lead cultures, 6-month follow-up including outpatient visit with TTE | accuracy 86% (78–92%), sensitivity 84% (71–97%), specificity 88% (80–95%), NPV 93% (86–99%), PPV 74% (60–89%) diagnostic criteria including 99mTc-HMPAO-SPECT/CT: accuracy 88% (81–94%), sensitivity 87% (75–99%), specificity 89% (82–96%), NPV 94% (89–100%), PPV 77% (63–91%) | high |
| Erba, P. A. | retrospective | 63 | lead cultures, Duke criteria, follow-up | accuracy 96.8% (88–99.4%), sensitivity 93.7% (83.9–98%), specificity 100% (92.8–100%), PPV 100% (92.8–100%), NPV 93.9% (84.1–98.1%) | high |
| Holcman, K. | prospective | 103 | multidisciplinary expert team, lead cultures, 17.48 ± 11.90-month follow-up including outpatient visit with TTE | in-hospital mortality OR 19.6 (1.02–374.3), complications HR 5.9 (2.27–15.2), complete hardware removal HR 4.3 2.07–19.08 | high |
| 18F-FDG PET/CT | 99mTc-HMPAO-SPECT/CT | |
|---|---|---|
| Patient preparation | at least 4 h fasting, 24 h low-carbohydrate and high-fat diet, in some protocols intravenous heparin or calcium channel blockers are administered prior to the acquisition | tracer preparation involving isolation of autologous leucocytes, incubation with tracer HMPAO and radioisotope 99mTc, followed by intravenous autologous radiolabeled leucocytes administration |
| Duration | Short—single acquisition 60 min after tracer injection | Long—24 h, including tracer injection and early, delayed and late acquisitions |
| Radiation dose | 2.5–5.0 Megabecquerels/kilogram, that is 175–350 Megabecquerels in a 70-kg standard adult | 370–740 Megabecquerels |
| Quantification | possible | limited |
| Anatomical resolution | good | sufficient |
| Availability | different across countries | different across countries |
| Diagnostic properties -direct comparison -cumulative data | -more sensitive 30.8–100% sensitivity, 62.5–100% specificity, 75–100% NPV, 66–100% PPV | -more specific 60–93.7% sensitivity, 88–100% specificity, 85–93.9% NPV, 74–100% PPV |
| Prognostic value | ‘Cold Closed Pocket’ is associated with mortality | positive result for CDRIE is associated with increased in-hospital mortality and complication rates |
| Quantity of supporting literature evidence | moderate | limited |
| Limitations | - myocardial and respiratory artifacts - inflammatory lesions difficult to differentiate from infection sites - limited detection of smaller vegetations along CIED leads- better properties for diagnosing LDI than CDRIE | - metallic artifacts - non-specific activity in the bowel as a result of hepatic HMPAO excretion - limited detection of smaller vegetations |
| Interference from ongoing steroid treatment | probable | no evidence |
| Interference from ongoing antimicrobial treatment | probable | probable |
| Contraindications | -pregnancy -uncontrolled diabetes mellites | -pregnancy -neutropenia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holcman, K.; Rubiś, P.; Stępień, A.; Graczyk, K.; Podolec, P.; Kostkiewicz, M. The Diagnostic Value of 99mTc-HMPAO-Labelled White Blood Cell Scintigraphy and 18F-FDG PET/CT in Cardiac Device-Related Infective Endocarditis—A Systematic Review. J. Pers. Med. 2021, 11, 1016. https://doi.org/10.3390/jpm11101016
Holcman K, Rubiś P, Stępień A, Graczyk K, Podolec P, Kostkiewicz M. The Diagnostic Value of 99mTc-HMPAO-Labelled White Blood Cell Scintigraphy and 18F-FDG PET/CT in Cardiac Device-Related Infective Endocarditis—A Systematic Review. Journal of Personalized Medicine. 2021; 11(10):1016. https://doi.org/10.3390/jpm11101016
Chicago/Turabian StyleHolcman, Katarzyna, Paweł Rubiś, Agnieszka Stępień, Katarzyna Graczyk, Piotr Podolec, and Magdalena Kostkiewicz. 2021. "The Diagnostic Value of 99mTc-HMPAO-Labelled White Blood Cell Scintigraphy and 18F-FDG PET/CT in Cardiac Device-Related Infective Endocarditis—A Systematic Review" Journal of Personalized Medicine 11, no. 10: 1016. https://doi.org/10.3390/jpm11101016