An Exploratory Association Analysis of ABCB1 rs1045642 and ABCB1 rs4148738 with Non-Major Bleeding Risk in Atrial Fibrillation Patients Treated with Dabigatran or Apixaban
Abstract
:1. Introduction
2. Materials and Methods
3. Results
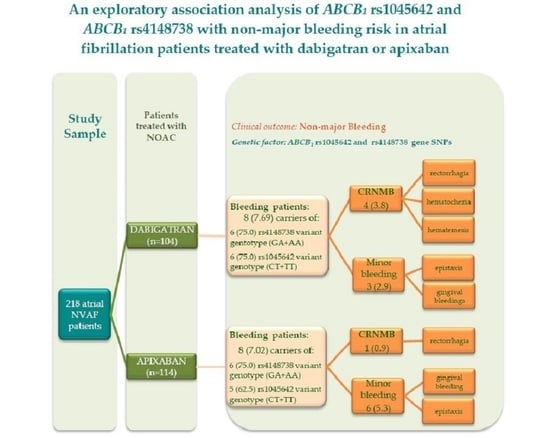
3.1. Characterization of Patients Treated with NOACs
3.2. The Association between ABCB1 SNP and Odds of Bleedings
3.3. Haplotype Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gadsboll, K.; Staerk, L.; Fosbol, E.L.; Sindet-Pedersen, C.; Gundlund, A.; Lip, G.Y.H.; Gislason, G.H.; Olesen, J.B. Increased use of oral anticoagulants in patients with atrial fibrillation: Temporal trends from 2005 to 2015 in Denmark. Eur. Heart J. 2017, 38, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Huiart, L.; Ferdynus, C.; Renoux, C.; Beaugrand, A.; Lafarge, S.; Bruneau, L.; Suissa, S.; Maillard, O.; Ranouil, X. Trends in initiation of direct oral anticoagulant therapies for atrial fibrillation in a national population-based cross-sectional study in the French health insurance databases. BMJ Open 2018, 8, e018180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boriani, G.; Proietti, M.; Laroche, C.; Fauchier, L.; Marin, F.; Nabauer, M.; Potpara, T.; Dan, G.A.; Kalarus, Z.; Diemberger, I.; et al. Contemporary stroke prevention strategies in 11 096 European patients with atrial fibrillation: A report from the EURObservational Research Programme on Atrial Fibrillation (EORP-AF) Long-Term General Registry. EP Eur. 2017, 20, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.-C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. The Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC). EHJ 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [Green Version]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. JACC 2019, 140, e125–e151. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Hylek, E.; Ko, D.; Cove, C.L. Gaps in translation from trials to practice: Non-Vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in atrial fibrillation. Thromb. Haemost. 2014, 111, 783–788. [Google Scholar] [CrossRef]
- Powell, J.R. Are new oral anticoagulant dosing recommendations optimal for all patients? JAMA 2015, 313, 1013–1014. [Google Scholar] [CrossRef]
- Douxfils, J.; Mullier, F.; Dogne, J.M. Dose tailoring of dabigatran etexilate: Obvious or excessive? Expert Opin. Drug Saf. 2015, 14, 1283–1289. [Google Scholar] [CrossRef]
- European Medicines Agency. Summary of Products Characteristics Pradaxa. Available online: https://ec.europa.eu/health/documents/community-register/2019/20190513144929/anx_144929_en.pdf (accessed on 30 July 2020).
- Summary of Product Characteristics Eliquis (Apixaban). Available online: https://www.ema.europa.eu/en/documents/product-information/eliquis-epar-product-information_en.pdf (accessed on 30 July 2020).
- Gong, I.Y.; Kim, R.B. Importance of pharmacokinetic profile and variability as determinants of dose and response to dabigatran, rivaroxaban, and apixaban. Can. J. Cardiol. 2013, 29 (Suppl. S7), S24–S33. [Google Scholar] [CrossRef]
- Gladding, P. Clinical applications of pharmacogenetics: Present and near future. Clin. J. Med. 2013, 80, 477–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pare, G.; Eriksson, N.; Lehr, T.; Connolly, S.; Eikelboom, J.; Ezekowitz, M.D.; Siegbahn, A.; Syvanen, A.-C.; Wadelius, C.; Wadelius, M.; et al. Genetic determinants of dabigatran plasma levels and their relation to bleeding. Circulation 2013, 127, 1404–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouin-Thibault, I.; Delavenne, X.; Blanchard, A.; Siguret, V.; Salem, J.E.; Narjoz, C.; Loriot, M.A. Interindividual variability in dabigatran and rivaroxaban exposure: Contribution of ABCB1 genetic polymorphisms and interaction with clarithromycin. J. Thromb. Haemost. 2017, 15, 273–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stöllberger, C.; Finsterer, J. Relevance of P-glycoprotein in stroke prevention with dabigatran, rivaroxaban, and apixaban. Herz 2015, 40 (Suppl. S2), 140–145. [Google Scholar] [CrossRef]
- Vranckx, P.; Valgimigli, M.; Heidbuchel, H. The significance of drug–drug and drug–food interactions of oral anticoagulation. Arrhythmia Electrophysiol. Rev. 2018, 7, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.F. Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica 2008, 38, 802–832. [Google Scholar] [CrossRef]
- Brambila-Tapia, A.J.L. MDR1 (ABCB1) polymorphisms: Functional effects and clinical implications. Rev. Investig. Clin. 2013, 65, 445–454. [Google Scholar]
- Group, Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Cockcroft, D.W.; Gaultd, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. The Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef]
- Hellenbart, E.L.; Faulkenberg, K.D.; Finks, S. Evaluation of bleeding in patients receiving direct oral anticoagulants. Vasc. Heal. Risk Manag. 2017, 13, 325–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wigginton, J.E.; Cutler, D.J.; Abecasis, G.R. A note on exact tests of Hardy-Weinberg equilibrium. Am. J. Hum. Genet. 2005, 76, 887–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warnes, G.; Gorjanc, G.; Leisch, F.; Man, M. Package ‘Genetics’: Population Genetics. 2019 R package Version 1.3.8.1.2. Available online: https://CRAN.R-project.org/package=genetics (accessed on 20 July 2020).
- Ensembld Data Base. Available online: http://www.ensembl.org/Homo_sapiens/Variation/Population (accessed on 30 March 2020).
- Butt, J.H.; Li, A.; Xian, Y.; Peterson, E.D.; Garcia, D.; Torp-Pedersen, C.; Køber, L.; Fosbøl, E.L. Direct Oral Anticoagulant- versus Vitamin K Antagonist-related Gastrointestinal Bleeding: Insights from a nationwide cohort. Am. Heart J. 2019, 216, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, V.V.; Calina, D.; Tsarouhas, K.; Pivnik, A.V.; Sergievich, A.A.; Kodintsev, V.V.; Filatova, E.A.; Ozcagli, E.; Docea, A.O.; Arsene, A.L.; et al. A guide to acquired vitamin K coagulophathy diagnosis and treatment: The Russian perspective. DARU J. Pharm. Sci. 2019, 25, 10. [Google Scholar] [CrossRef] [Green Version]
- Pollack, C.V., Jr.; Reilly, P.A.; Eikelboom, J.; Glund, S.; Verhamme, P.; Bernstein, R.A.; Dubiel, R.; Huisman, M.V.; Hylek, E.M.; Kam, C.-W.; et al. Idarucizumab for dabigatran reversal. N. Engl. J. Med. 2015, 377, 511–520. [Google Scholar] [CrossRef] [Green Version]
- Sartori, M.; Cosmi, B. Andexanet alfa to reverse the anticoagulant activity of factor Xa inhibitors: A review of design, development and potential place in therapy. J. Thromb. Thrombolysis 2018, 45, 345–352. [Google Scholar] [CrossRef]
- Healey, J.S.; Eikelboom, J.; Douketis, J.; Wallentin, L.; Oldgren, J.; Yang, S.; Themeles, E.; Heidbuchel, H.; Avezum, A.; Reilly, P.; et al. Periprocedural bleeding and thromboembolic events with dabigatran compared withwarfarin: Results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 2012, 126, 343–348. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, C.T.; Kiernan, T.J.; Yan, B.P. The genetic basis of antiplatelet and anticoagulant therapy: A pharmacogenetic review of newer antiplatelets (clopidogrel, prasugrel and ticagrelor) and anticoagulants (dabigatran, rivaroxaban, apixaban and edoxaban). Expert Opin. Drug Metab. Toxicol. 2017, 13, 725–739. [Google Scholar] [CrossRef]
- Cullell, N.; Carrera, C.; Muiño, E.; Torres, N.; Krupinski, J.; Fernandez-Cadenas, I. Pharmacogenetic studies with oral anticoagulants. Genome-Wide association studies in vitamin K antagonist and direct oral anticoagulants. Oncotarget 2018, 9, 29238–29258. [Google Scholar] [CrossRef]
- Roşian, A.N.; Roşian, Ş.H.; Kiss, B.; Ştefan, M.G.; Trifa, A.P.; Ober, C.; Anchidin, O.; Buzoianu, A.D. Interindividual Variability of Apixaban Plasma Concentrations: Influence of Clinical and Genetic Factors in a Real-Life Cohort of Atrial Fibrillation Patients. Genes 2020, 11, 438. [Google Scholar] [CrossRef]
- Roşian, A.N.; Roşian, S.H.; Kiss, B.; Ştefan, G.M.; Trifa, A.P.; Ober, C.D.; Bangău, V.; Mada, C.; Gocan, C.P.; Niţă, T.; et al. Genotype-Phenotype Correlation for Dabigatran in Patients with Non-Valvular Atrial Fibrillation (A Single Centre Research). HVM Bioflux (Rom.) 2020, 12, 123–129. [Google Scholar]
- Dimatteo, C.; D’Andrea, G.; Vecchione, G.; Paoletti, O.; Cappucci, F.; Tiscia, G.L.; Margaglione, M. Pharmacogenetics of dabigatran etexilate interindividual variability. Thromb. Res. 2016, 144, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sychev, D.A.; Levanov, A.; Shelekhova, T.; Bochkov, P.O.; Denisenko, N.; Ryzhikova, K.; Bogoslovsky, T. The impact of ABCB1 (rs1045642 and rs4148738) and CES1 (rs2244613) gene polymorphisms on dabigatran equilibrium peak concentration in patients after total knee arthroplasty. Pharm. Pers. Med. 2018, 11, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Dimatteo, C.; D’Andrea, G.; Vecchione, G.; Paoletti, O.; Tiscia, G.L.; Santacroce, R.; Correale, M.; Brunetti, N.; Grandone, E.; Testa, S.; et al. ABCB1 SNP rs4148738 modulation of apixaban interindividual variability. Thromb. Res. 2016, 145, 24–26. [Google Scholar] [CrossRef]
- Kryukov, A.V.; Sychev, D.A.; Andreev, D.A.; Ryzhikova, K.A.; Grishina, E.A.; Ryabova, A.V.; Loskutnikov, M.A.; Smirnov, V.V.; Konova, O.D.; Matsneva, I.A.; et al. Influence of ABCB1 and CYP3A5 gene polymorphisms on pharmacokinetics of apixaban in patients with atrial fibrillation and acute stroke. Pharm. Pers. Med. 2018, 11, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Nantsupawat, T.; Soontrapa, S.; Nantsupawat, N.; Sotello, D.; Klomjit, S.; Adabag, S.; Perez-Verdia, A. Risk factors and prevention of dabigatran-related gastrointestinal bleeding in patients with atrial fibrillation. J. Arrhythmia 2017, 34, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Green, L.; Tan, J.; Morris, J.K.; Alikhan, R.; Curry, N.; Everington, T.; McLean, R.; Saja, K.; Stanworth, S.; Tait, C.; et al. A three-year prospective study of the presentation and clinical outcomes of major bleeding episodes associated with oral anticoagulant use in the UK (ORANGE study). Haematologica 2018, 103, 738–745. [Google Scholar] [CrossRef]
- Miller, C.S.; Dorreen, A.; Martel, M.; Huynh, T.; Barkun, A.N. Risk of Gastrointestinal Bleeding in Patients Taking Non–Vitamin K Antagonist Oral Anticoagulants: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2017, 15, 1674–1683. [Google Scholar] [CrossRef] [Green Version]
- Bahit, M.C.; Lopes, R.D.; Wojdyla, D.M.; Held, C.; Hanna, M.; Vinereanu, D.; Hylek, E.M.; Verheugt, F.; Goto, S.; Alexander, J.H.; et al. Non-Major bleeding with apixaban versus warfarin in patients with atrial fibrillation. Heart 2017, 103, 623–628. [Google Scholar] [CrossRef]
- Sengupta, N.; Marshall, A.L.; Jones, B.A.; Ham, S.; Tapper, E.B. Rebleeding vs. thromboembolism after hospitalization for gastrointestinal bleeding in patients on direct oral anticoagulants. Clin. Gastroenterol. Hepatol. 2018, 16, 1893–1900. [Google Scholar] [CrossRef] [Green Version]
- Eikelboom, J.W.; Wallentin, L.; Connolly, S.J.; Ezekowitz, M.; Healey, J.S.; Oldgren, J.; Yang, S.; Alings, M.; Kaatz, S.; Hohnloser, S.H.; et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: An analysis of the. Circulation 2011, 123, 2363–2372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueshima, S.; Hira, D.; Fujii, R.; Kimura, Y.; Tomitsuka, C.; Yamane, T.; Tabuchi, Y.; Ozawa, T.; Itoh, H.; Horie, M.; et al. Impact of ABCB1, ABCG2, and CYP3A5 polymorphisms on plasma trough concentrations of apixaban in Japanese patients with atrial fibrillation. Pharm. Genom. 2017, 27, 329–336. [Google Scholar] [CrossRef]
- Marzolini, C.; Paus, E.; Buclin, T.; Kim, R.B. Polymorphisms in human MDR1 (P-glycoprotein): Recent advances and clinical relevance. Clin. Pharm. Ther. 2004, 75, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Andreotti, F.; Fauchier, L.; Huber, K.; Hylek, E.; Knight, E.; Lane, D.A.; Levi, M.; Marin, F.; Palareti, G.; et al. Bleeding risk assessment and management in atrial fibrillation patients: A position document from the European Heart Rhythm Association, endorsed by the European Society of Cardiology Working Group on Thrombosis. Europace 2011, 13, 723–746. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.S.; Noseworthy, P.A.; Yao, X.; Sangaralingham, L.R.; Shah, N.D. Gastrointestinal safety of direct oral anticoagulants: A large population-based study. Gastroenterology 2016, 152, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Abraham, N.S.; Singh, S.; Alexander, G.C.; Heien, H.; Haas, L.R.; Crown, W.; Shah, N.D. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: Population based cohort study. BMJ 2015, 350, h1857. [Google Scholar] [CrossRef] [Green Version]
- Deitelzweig, S.; Farmer, C.; Luo, X.; Li, X.; Vo, L.; Mardekian, J.; Fahrbach, K.; Ashaye, A. Comparison of major bleeding risk in patients with non-valvular atrial fibrillation receiving direct oral anticoagulants in the real-world setting: A network meta-analysis. Curr. Med. Res. Opin. 2018, 34, 487–498. [Google Scholar] [CrossRef]
- Huisman, M.V.; Rothman, K.J.; Paquette, M.; Teutsch, C.; Diener, H.C.; Dubner, S.J.; Halperin, J.L.; Ma, C.S.; Zint, K.; Elsaesser, A.; et al. Two-Year follow-up of patients treated with dabigatran for stroke prevention inatrial fibrillation: GLORIA-AF Registry. Am. Heart J. 2018, 198, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, M.S.; van Hulst, M.; Campmans, Z.; Tieleman, R.G. Inappropriate non-vitamin K antagonist oral anticoagulants prescriptions: Be cautious with dose reductions. Neth. Heart J. 2019, 27, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Ibáñez, L.; Sabaté, M.; Vidal, X.; Ballarin, E.; Rottenkolber, M.; Schmiedl, S.; Heeke, A.; Huerta, C.; Martin Merino, E.; Montero, D.; et al. Incidence of Direct Oral Anticoagulant use in patients with non-valvular atrial fibrillation and characteristics of users in six European countries (2008–2015): A cross-national drug utilization study. Br. J. Clin. Pharm. 2019, 85, 2524–2539. [Google Scholar] [CrossRef] [Green Version]
- Sylvester, K.W.; Ting, C.; Lewin, A.; Collins, P.; Fanikos, J.; Goldhaber, S.Z.; Connors, J.M. Expanding anticoagulation management services to include direct oral anticoagulants. J. Thromb. Thrombolysis 2018, 45, 274–280. [Google Scholar] [CrossRef] [PubMed]

| Demographic and Clinical Variables | NOAC Therapy (n = 218) | DABIGATRAN (n1 = 104) | APIXABAN (n2 = 114) | p-Value |
|---|---|---|---|---|
| Age (years), mean ± SD | 70.94 ± 9.04 | 70.89 ± 8.85 | 70.97 ± 9.24 | 0.949 |
| Male, fa (%) | 113 (51.83) | 55 (52.88) | 58 (50.88) | 0.767 |
| AF type, fa (%) | 0.980 | |||
| paroxysmal | 74 (33.94) | 36 (34.62) | 38 (33.33) | |
| persistent | 51 (23.39) | 24 (23.08) | 27 (23.68) | |
| permanent | 93 (42.66) | 44 (42.31) | 49 (42.98) | |
| BMI (kg/m2), mean ± SD | 28.06 ± 4.32 | 28.83 ± 4.11 | 27.35 ± 4.41 | 0.012 * |
| CHA2DS2-VASc score ≥ 2 | 199 (91.28) | 95 (91.35) | 104 (91.23) | 0.975 |
| HAS-BLED score ≥ 3 | 29 (13.30) | 17 (16.35) | 12 (10.53) | 0.206 |
| CrCl ml/min/1.72 m2, mean ± SD | 79.48 ± 27.24 | 81.57 ± 28.53 | 77.58 ± 25.98 | 0.281 |
| LADi (mm/ m2), median (Q1; Q3) | 22.78 (20.72; 25.75) | 22.12 (20.08; 25.02) | 23.38 (21.10; 26.25) | 0.016 * |
| LAVi (ml/ m2), median (Q1; Q3) | 33.39 (30.55; 40.01) | 33.44 (30.20; 38.92) | 35.57 (31.62; 40.37) | 0.184 |
| Prior VKA, fa (%) | 102 (46.79) | 64 (61.54) | 38 (33.33) | <0.0001 ** |
| P-gp inhibitors, fa (%) | 54 (24.77) | 24 (23.08) | 30 (26.32) | 0.580 |
| Antiplatelet. fa (%) | 33 (15.14) | 14 (13.46) | 19 (16.67) | 0.510 |
| NSAIDs, fa (%) | 16 (7.34) | 11 (10.58) | 5 (4.39) | 0.080 |
| Bleeding, fa (%) | 16 (7.34) | 8 (7.69) | 8 (7.02) | 0.849 |
| SNPs | NOAC Therapy | DABIGATRAN | APIXABAN | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (n = 218) | Total (n1 = 104) | Bleeding (n1 = 8) | No Bleeding (n2 = 96) | p-Value | Total (n2 = 114) | Bleeding (n1 = 8) | No Bleeding (n2 = 106) | p-Value | |
| ABCB1 rs1045642 C > T MAF CEU* = 43.4 | pHWE = 0.230 | pHWE = 0.710 | |||||||
| MAF (95% CI) | 46.56 (41.80; 51.37) | 43.27 (36.44; 50.30) | 43.75 (19.75; 70.12) | 43.23 (36.12; 50.56) | - | 49.56 (42.90; 56.39) | 37.50 (15.20; 64.56) | 50.47 (43.54; 7.39) | - |
| Genotype | 0.965 | 0.325 | |||||||
| CC | 60 (27.52) | 30 (28.84) | 2 (25) | 28 (29.16) | 30 (26.31) | 3 (37.5) | 27 (25.47) | ||
| CT | 113 (51.83) | 58 (55.76) | 5 (62.5) | 53 (55.2) | 55 (48.24) | 4 (50) | 51 (48.11) | ||
| TT | 45 (20.64) | 16 (15.38) | 1 (12.5) | 15 (15.62) | 29 (25.43) | 1 (12.5) | 28 (26.4) | ||
| ABCB1 rs4148738 G > A MAF CEU* = 46.0 | pHWE = 0.160 | pHWE = 0.850 | |||||||
| MAF (95% CI) | 43.35 (38.64; 48.15) | 41.35 (34.58; 48.36) | 50.00 (24.65; 75.35) | 40.63 (33.61; 47.93) | - | 45.18 (38.60; 1.88) | 50.00 (24.65; 75.35) | 44.81 (37.99; 51.77) | - |
| Genotype | 0.428 | 0.691 | |||||||
| GG | 67 (30.73) | 32 (30.76) | 2 (25) | 30 (31.25) | 35 (30.70) | 2 (25) | 33 (31.13) | ||
| GA | 113 (51.83) | 58 (55.76) | 4 (50) | 54 (56.25) | 55 (48.24) | 4 (50) | 51 (48.11) | ||
| AA | 38 (17.43) | 14 (13.46) | 2 (25) | 12 (12.5) | 24 (21.05) | 2 (25) | 22 (20.75) | ||
| SNPs | Tested Genetic Models | Genotype | Adjusted OR (95% CI) | p-Value |
|---|---|---|---|---|
| ABCB1 rs1045642 C > T | Codominant | CC | Reference | 0.685 |
| CT | 0.95 (0.30; 2.98) | |||
| TT | 0.52 (0.09; 2.80) | |||
| Dominant | CC | Reference | 0.736 | |
| CT+TT | 0.83 (0.27; 2.45) | |||
| Recessive | CC+CT | Reference | 0.386 | |
| TT | 0.53 (0.12; 2.41) | |||
| Overdominant | TT+CC | Reference | 0.723 | |
| CT | 1.20 (0.43; 3.37) | |||
| ABCB1 rs4148738 G > A | Codominant | GG | Reference | 0.688 |
| GA | 1.19 (0.35; 4.13) | |||
| AA | 1.88 (0.44; 8.04) | |||
| Dominant | GG | Reference | 0.599 | |
| GA +AA | 1.36 (0.42; 4.38) | |||
| Recessive | GG + GA | Reference | 0.414 | |
| AA | 1.68 (0.51; 5.57) | |||
| Overdominant | GG+ AA | Reference | 0.867 | |
| GA | 0.92 (0.33; 2.54) |
| Bleeding (n1 = 16) | No Bleeding (n2 = 202) | Unadjusted OR (95% CI) | p-Value | |
|---|---|---|---|---|
| Age (>70 years vs. ≤ 70) | 12 (75.00) | 104 (51.49) | 2.83 (0.95; 10.38) | 0.080 |
| Sex (male vs. female) | 6 (37.50) | 107 (53.00) | 0.53 (0.18; 1.49) | 0.239 |
| Diabetes mellitus (yes vs. no) | 7 (43.75) | 50 (24.75) | 2.36 (0.81; 6.67) | 0.104 |
| Hypertension (yes vs. no) | 14 (87.50) | 167 (82.67) | 1.47 (0.39; 9.61) | 0.622 |
| Stroke/TIA history (yes vs. no) | 5 (31.32) | 28 (13.86) | 2.82 (0.84; 8.42) | 0.072 |
| Ischemic heart disease (yes vs. no) | 3 (18.75) | 45 (22.28) | 0.81 (0.18; 2.63) | 0.744 |
| Peripheral vascular disease (yes vs. no) | 2 (12.50) | 22 (10.89) | 1.17 (0.18; 4.56) | 0.843 |
| Heart failure (yes vs. no) | 1 (6.25) | 32 (15.84) | 0.35 (0.02; 1.84) | 0.323 |
| Chronic kidney disease (yes vs. no) | 4 (25.00) | 53 (26.24) | 0.94 (0.25; 2.82) | 0.914 |
| Gastritis/peptic ulcer (yes vs. no) | 0 (0.00) | 6 (3.0) | ND | 0.993 |
| Smoking # (yes vs. no) | 2 (12.50) | 18 (9.5) | 1.37 (0.20; 5.43) | 0.696 |
| Alcohol consumption #(yes vs. no) | 2 (12.50) | 8 (4.2) | 3.25 (0.46; 14.58) | 0.159 |
| Haplotypes | Haplotype-Frequencies (%) in NOAC Patients | Hap-Score Statistics (a) | p (b) | Unadjusted OR (c), 95% CI (Lower; Upper Limit) | ||
|---|---|---|---|---|---|---|
| Overall Sample | with Bleedings | without Bleedings | ||||
| C-G | 48.41 | 50.00 | 48.32 | 0.13 | 0.894 | 1.00 (Reference) |
| T-A | 38.32 | 40.63 | 38.17 | 0.23 | 0.816 | 1.04 (0.47; 2.31) |
| T-G | 8.25 | 0.00 | 8.86 | −1.68 | 0.092 | <0.00001 |
| C-A | 5.03 | 9.36 | 4.65 | 1.33 | 0.183 | 1.91 (0.53; 6.93) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roşian, A.-N.; Iancu, M.; Trifa, A.P.; Roşian, Ş.H.; Mada, C.; Gocan, C.P.; Niţă, T.; Istratoaie, S.; Boarescu, P.-M.; Buzoianu, A.D. An Exploratory Association Analysis of ABCB1 rs1045642 and ABCB1 rs4148738 with Non-Major Bleeding Risk in Atrial Fibrillation Patients Treated with Dabigatran or Apixaban. J. Pers. Med. 2020, 10, 133. https://doi.org/10.3390/jpm10030133
Roşian A-N, Iancu M, Trifa AP, Roşian ŞH, Mada C, Gocan CP, Niţă T, Istratoaie S, Boarescu P-M, Buzoianu AD. An Exploratory Association Analysis of ABCB1 rs1045642 and ABCB1 rs4148738 with Non-Major Bleeding Risk in Atrial Fibrillation Patients Treated with Dabigatran or Apixaban. Journal of Personalized Medicine. 2020; 10(3):133. https://doi.org/10.3390/jpm10030133
Chicago/Turabian StyleRoşian, Adela-Nicoleta, Mihaela Iancu, Adrian Pavel Trifa, Ştefan Horia Roşian, Cristina Mada, Cornelia Paula Gocan, Teodora Niţă, Sabina Istratoaie, Paul-Mihai Boarescu, and Anca Dana Buzoianu. 2020. "An Exploratory Association Analysis of ABCB1 rs1045642 and ABCB1 rs4148738 with Non-Major Bleeding Risk in Atrial Fibrillation Patients Treated with Dabigatran or Apixaban" Journal of Personalized Medicine 10, no. 3: 133. https://doi.org/10.3390/jpm10030133