Human AI Teaming for Coronary CT Angiography Assessment: Impact on Imaging Workflow and Diagnostic Accuracy
Abstract
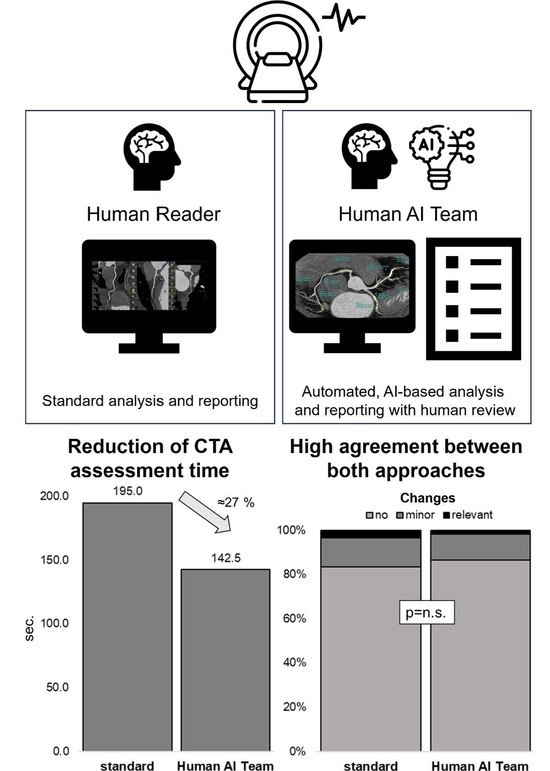
:1. Introduction
2. Materials and Methods
2.1. Image Acquisition
2.2. Image Analysis
2.3. AI Analysis
2.4. Statistics
3. Results
3.1. Patient Characteristics
3.2. Workflow Optimization
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 24 May 2023).
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Recent-Onset Chest Pain of Suspected Cardiac Origin: Assessment and Diagnosis; National Institute for Health and Care Excellence: Manchester, UK, 2016. [Google Scholar]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 144, e368–e454. [Google Scholar] [CrossRef] [PubMed]
- DISCHARGE Trial Group; Maurovich-Horvat, P.; Bosserdt, M.; Kofoed, K.F.; Rieckmann, N.; Benedek, T.; Donnelly, P.; Rodriguez-Palomares, J.; Erglis, A.; Stechovsky, C.; et al. CT or Invasive Coronary Angiography in Stable Chest Pain. N. Engl. J. Med. 2022, 386, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Fortner, P.; Emami, M.; Seitz, S.; Brado, M.; Guckel, F.; Sokiranski, R.; Sommer, A.; Frey, N.; Gorich, J.; et al. Factors influencing the safety of outpatient coronary CT angiography: A clinical registry study. BMJ Open 2022, 12, e058304. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.C.; Moss, A.J.; Dweck, M.; Adamson, P.D.; Alam, S.; Hunter, A.; Shah, A.S.V.; Pawade, T.; Weir-McCall, J.R.; Roditi, G.; et al. Coronary Artery Plaque Characteristics Associated with Adverse Outcomes in the SCOT-HEART Study. J. Am. Coll. Cardiol. 2019, 73, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Ovrehus, K.A.; Diederichsen, A.; Grove, E.L.; Steffensen, F.H.; Mortensen, M.B.; Jensen, J.M.; Mickley, H.; Nielsen, L.H.; Busk, M.; Sand, N.P.R.; et al. Reduction of Myocardial Infarction and All-Cause Mortality Associated to Statins in Patients without Obstructive CAD. JACC Cardiovasc. Imaging 2021, 14, 2400–2410. [Google Scholar] [CrossRef] [PubMed]
- Weir-McCall, J.R.; Williams, M.C.; Shah, A.S.V.; Roditi, G.; Rudd, J.H.F.; Newby, D.E.; Nicol, E.D. National Trends in Coronary Artery Disease Imaging. JACC Cardiovasc. Imaging 2023, 16, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Baessler, B.; Gotz, M.; Antoniades, C.; Heidenreich, J.F.; Leiner, T.; Beer, M. Artificial intelligence in coronary computed tomography angiography: Demands and solutions from a clinical perspective. Front. Cardiovasc. Med. 2023, 10, 1120361. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Huang, L.; Qu, M.; Chen, B.; Wang, G. Artificial Intelligence in Coronary CT Angiography: Current Status and Future Prospects. Front. Cardiovasc. Med. 2022, 9, 896366. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.D.; Marques, H.; Kumar, V.; Griffin, W.F.; Rahban, H.; Karlsberg, R.P.; Zeman, R.K.; Katz, R.J.; Earls, J.P. CT Evaluation by Artificial Intelligence for Atherosclerosis, Stenosis and Vascular Morphology (CLARIFY): A Multi-center, international study. J. Cardiovasc. Comput. Tomogr. 2021, 15, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Khasanova, E.; Indraratna, P.; Miranda, P.; Takagi, H.; Chuang, M.-y.; Park, K.-H.; Sellers, S.; Leipsic, J. Head to Head comparison reproducibility and inter-reader agreement of an AI based coronary stenosis algorithm vs level 3 readers. J. Cardiovasc. Comput. Tomogr. 2022, 16, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Leipsic, J.; Abbara, S.; Achenbach, S.; Cury, R.; Earls, J.P.; Mancini, G.J.; Nieman, K.; Pontone, G.; Raff, G.L. SCCT guidelines for the interpretation and reporting of coronary CT angiography: A report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J. Cardiovasc. Comput. Tomogr. 2014, 8, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Austen, W.G.; Edwards, J.E.; Frye, R.L.; Gensini, G.G.; Gott, V.L.; Griffith, L.S.; McGoon, D.C.; Murphy, M.L.; Roe, B.B. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975, 51, 5–40. [Google Scholar] [CrossRef] [PubMed]
- Gülsün, M.A.; Funka-Lea, G.; Sharma, P.; Rapaka, S.; Zheng, Y. Coronary centerline extraction via optimal flow paths and CNN path pruning. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention-MICCAI 2016: 19th International Conference, Athens, Greece, 17–21 October 2016; pp. 317–325. [Google Scholar]
- Xu, L.; He, Y.; Luo, N.; Guo, N.; Hong, M.; Jia, X.; Wang, Z.; Yang, Z. Diagnostic Accuracy and Generalizability of a Deep Learning-Based Fully Automated Algorithm for Coronary Artery Stenosis Detection on CCTA: A Multi-Centre Registry Study. Front. Cardiovasc. Med. 2021, 8, 707508. [Google Scholar] [CrossRef] [PubMed]
- Griffin, W.F.; Choi, A.D.; Riess, J.S.; Marques, H.; Chang, H.J.; Choi, J.H.; Doh, J.H.; Her, A.Y.; Koo, B.K.; Nam, C.W.; et al. AI Evaluation of Stenosis on Coronary CTA, Comparison with Quantitative Coronary Angiography and Fractional Flow Reserve: A CREDENCE Trial Substudy. JACC Cardiovasc. Imaging 2023, 16, 193–205. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (FDA); Healthcare Products Regulatory Agency. Good Machine Learning Practice for Medical Device Development: Guiding Principles; U.S. Food and Drug Administration (FDA): Silver Spring, MD, USA, 2021.
- Chen, C.; Li, X.; Su, Y.; You, Z.; Wan, R.; Hong, K. Adherence with cardiovascular medications and the outcomes in patients with coronary arterial disease: “Real-world” evidence. Clin. Cardiol. 2022, 45, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Naderi, S.H.; Bestwick, J.P.; Wald, D.S. Adherence to drugs that prevent cardiovascular disease: Meta-analysis on 376,162 patients. Am. J. Med. 2012, 125, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.L.; McRae, D.; Sheils, E.; McDonnell, B.J.; Khan, I.; James, D.H. The effect of visual interventions on illness beliefs and medication adherence for chronic conditions: A scoping review of the literature and mapping to behaviour change techniques (BCTs). Res. Soc. Adm. Pharm. 2022, 18, 3239–3262. [Google Scholar] [CrossRef] [PubMed]
- Feger, S.; Elzenbeck, L.; Rieckmann, N.; Marek, A.; Dreger, H.; Beling, M.; Zimmermann, E.; Rief, M.; Chow, B.J.W.; Maurovich-Horvath, P.; et al. Effect of Computed Tomography Versus Invasive Coronary Angiography on Statin Adherence: A Randomized Controlled Trial. JACC Cardiovasc. Imaging 2021, 14, 1480–1483. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Luo, N.; Xu, L.; Cao, J.; Guo, N.; He, Y.; Hong, M.; Jia, X.; Wang, Z.; Yang, Z. Artificial intelligence stenosis diagnosis in coronary CTA: Effect on the performance and consistency of readers with less cardiovascular experience. BMC Med. Imaging 2022, 22, 28. [Google Scholar] [CrossRef] [PubMed]





| Standard | Human AI Team | ||
|---|---|---|---|
| Age (years) | 62.5 (53.8–73.3) | 62.1 (55.3–71.8) | p = 0.63 |
| Sex (male) | 41 (68.3%) | 38 (63.3%) | p = 0.57 |
| BMI (kg/m2) | 27.8 (25.2–30.4) | 26.5 (24.7–30.0) | p = 0.49 |
| GFR (mL/min/1.73 m2) | 84.6 (75.0–94.0) | 79.4 (73.0–89.3) | p = 0.08 |
| Arterial Hypertension | 34 (56.7%) | 36 (60.0%) | p = 0.71 |
| Hyperlipidemia | 28 (46.7%) | 37 (61.7%) | p = 0.10 |
| Diabetes mellitus | 7 (11.7%) | 9 (15.0%) | p = 0.59 |
| Smoking | 10 (16.7%) | 3 (5.0%) | p = 0.04 |
| Family History of CAD | 36 (60.0%) | 33 (55.0%) | p = 0.58 |
| Heart Rate (/min) | 62.0 (58.5–65.0) | 60.0 (55.0–66.5) | p = 0.25 |
| Agatston Score | 17.7 (0.0–128.1) | 43.2 (0.0–212.6) | p = 0.60 |
| CAD-RADS | p = 0.87 | ||
| 0/1 | 17 (28.3%) | 17 (28.3%) | |
| 2 | 25 (41.7%) | 29 (48.3%) | |
| 3 | 13 (21.7%) | 8 (13.3%) | |
| 4A | 3 (5.0%) | 3 (5.0%) | |
| 4B | 1 (1.7%) | 1 (1.7%) | |
| 5 | 1 (1.7%) | 2 (3.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andre, F.; Fortner, P.; Aurich, M.; Seitz, S.; Jatsch, A.-K.; Schöbinger, M.; Wels, M.; Kraus, M.; Gülsün, M.A.; Frey, N.; et al. Human AI Teaming for Coronary CT Angiography Assessment: Impact on Imaging Workflow and Diagnostic Accuracy. Diagnostics 2023, 13, 3574. https://doi.org/10.3390/diagnostics13233574
Andre F, Fortner P, Aurich M, Seitz S, Jatsch A-K, Schöbinger M, Wels M, Kraus M, Gülsün MA, Frey N, et al. Human AI Teaming for Coronary CT Angiography Assessment: Impact on Imaging Workflow and Diagnostic Accuracy. Diagnostics. 2023; 13(23):3574. https://doi.org/10.3390/diagnostics13233574
Chicago/Turabian StyleAndre, Florian, Philipp Fortner, Matthias Aurich, Sebastian Seitz, Ann-Kathrin Jatsch, Max Schöbinger, Michael Wels, Martin Kraus, Mehmet Akif Gülsün, Norbert Frey, and et al. 2023. "Human AI Teaming for Coronary CT Angiography Assessment: Impact on Imaging Workflow and Diagnostic Accuracy" Diagnostics 13, no. 23: 3574. https://doi.org/10.3390/diagnostics13233574