Investigating the Feasibility of Predicting KRAS Status, Tumor Staging, and Extramural Venous Invasion in Colorectal Cancer Using Inter-Platform Magnetic Resonance Imaging Radiomic Features
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Information
2.2. RAS Mutation Information
2.3. MRI Acquisition
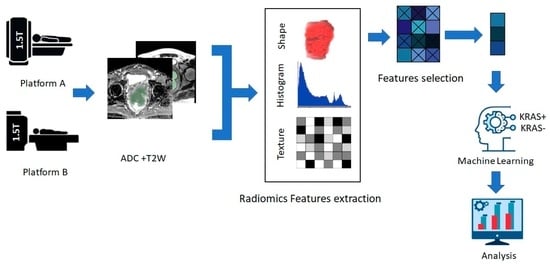
2.4. Radiomic Feature Extraction and Model Building
2.5. Machine Learning Model
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Feature Extraction, Feature Selection, and Radiomics Classifier
3.2.1. Radiomics Workflow
3.2.2. KRAS Mutation Status
3.2.3. Tumor Grades
3.2.4. Tumor Invasion to Mesorectal Fascia (MRF)
3.2.5. EMVI Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmermann, M.; Zouhair, A.; Azria, D.; Ozsahin, M. The epidermal growth factor receptor (EGFR) in head and neck cancer: Its role and treatment implications. Radiat. Oncol. 2006, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Mármol, I.; Sánchez-De-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Callum, K.G.; Whimster, F.; National Confidential Enquiry into Perioperative Deaths. Interventional Vascular Radiology and Interventional Neurovascular Radiology: A Report of the National Confidential Enquiry into Perioperative Deaths; NCEPOD: London, UK, 2000. [Google Scholar]
- Xu, Y.; Xu, Q.; Ma, Y.; Duan, J.; Zhang, H.; Liu, T.; Li, L.; Sun, H.; Shi, K.; Xie, S.; et al. Characterizing MRI features of rectal cancers with different KRAS status. BMC Cancer 2019, 19, 1111. [Google Scholar] [CrossRef]
- Guo, X.-F.; Yang, W.-Q.; Yang, Q.; Yuan, Z.-L.; Liu, Y.-L.; Niu, X.-H.; Xu, H.-B. Feasibility of MRI Radiomics for Predicting KRAS Mutation in Rectal Cancer. Curr. Med. Sci. 2020, 40, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shen, L.; Wang, Y.; Wang, J.; Zhang, H.; Xia, F.; Wan, J.; Zhang, Z. MRI Radiomics Signature as a Potential Biomarker for Predicting KRAS Status in Locally Advanced Rectal Cancer Patients. Front. Oncol. 2021, 11, 614052. [Google Scholar] [CrossRef]
- Al-Sukhni, E.; Milot, L.; Fruitman, M.; Beyene, J.; Victor, J.C.; Schmocker, S.; Brown, G.; McLeod, R.; Kennedy, E. Diagnostic Accuracy of MRI for Assessment of T Category, Lymph Node Metastases, and Circumferential Resection Margin Involvement in Patients with Rectal Cancer: A Systematic Review and Meta-analysis. Ann. Surg. Oncol. 2012, 19, 2212–2223. [Google Scholar] [CrossRef]
- Gollub, M.J.; Lakhman, Y.; McGinty, K.; Weiser, M.R.; Sohn, M.; Zheng, J.; Shia, J. Does Gadolinium-Based Contrast Material Improve Diagnostic Accuracy of Local Invasion in Rectal Cancer MRI? A Multireader Study. Am. J. Roentgenol. 2015, 204, W160–W167. [Google Scholar] [CrossRef] [PubMed]
- van Gijn, W.; Marijnen, C.A.; Nagtegaal, I.D.; Kranenbarg, E.M.-K.; Putter, H.; Wiggers, T.; Rutten, H.J.; Påhlman, L.; Glimelius, B.; van de Velde, C.J.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011, 12, 575–582. [Google Scholar] [CrossRef]
- Ma, X.; Shen, F.; Jia, Y.; Xia, Y.; Li, Q.; Lu, J. MRI-based radiomics of rectal cancer: Preoperative assessment of the pathological features. BMC Med. Imaging 2019, 19, 86. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Hu, B.; Gao, Y.; Chen, J.; Li, J. Development and validation of an MRI-based radiomic nomogram to distinguish between good and poor responders in patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiotherapy. Abdom. Radiol. 2020, 46, 1805–1815. [Google Scholar] [CrossRef]
- Oh, J.E.; Kim, M.J.; Lee, J.; Hur, B.Y.; Kim, B.; Kim, D.Y.; Baek, J.Y.; Chang, H.J.; Park, S.C.; Oh, J.H.; et al. Magnetic Resonance-Based Texture Analysis Differentiating KRAS Mutation Status in Rectal Cancer. Cancer Res. Treat. 2020, 52, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, G.; Kennedy, P.; Bane, O.; Kirmani, A.; El Homsi, M.; Stocker, D.; Said, D.; Mukherjee, P.; Gevaert, O.; Lewis, S.; et al. Precision of MRI radiomics features in the liver and hepatocellular carcinoma. Eur. Radiol. 2021, 32, 2030–2040. [Google Scholar] [CrossRef] [PubMed]
- Coppola, F.; Giannini, V.; Gabelloni, M.; Panic, J.; Defeudis, A.; Monaco, S.L.; Cattabriga, A.; Cocozza, M.A.; Pastore, L.V.; Polici, M.; et al. Radiomics and Magnetic Resonance Imaging of Rectal Cancer: From Engineering to Clinical Practice. Diagnostics 2021, 11, 756. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, Q.; Sun, H.; Liu, T.; Shi, K.; Wang, W. Could IVIM and ADC help in predicting the KRAS status in patients with rectal cancer? Eur. Radiol. 2018, 28, 3059–3065. [Google Scholar] [CrossRef]
- Lee, J.; Steinmann, A.; Ding, Y.; Lee, H.; Owens, C.; Wang, J.; Yang, J.; Followill, D.; Ger, R.; MacKin, D.; et al. Radiomics feature robustness as measured using an MRI phantom. Sci. Rep. 2021, 11, 3973. [Google Scholar] [CrossRef] [PubMed]
- Horng, H.; Singh, A.; Yousefi, B.; Cohen, E.A.; Haghighi, B.; Katz, S.; Noël, P.B.; Shinohara, R.T.; Kontos, D. Generalized ComBat harmonization methods for radiomic features with multi-modal distributions and multiple batch effects. Sci. Rep. 2022, 12, 4493. [Google Scholar] [CrossRef]
- Kim, T.-H.; Woo, S.; Han, S.; Suh, C.H.; Vargas, H.A. The Diagnostic Performance of MRI for Detection of Extramural Venous Invasion in Colorectal Cancer: A Systematic Review and Meta-Analysis of the Literature. Am. J. Roentgenol. 2019, 213, 575–585. [Google Scholar] [CrossRef]
- Liu, L.-H.; Lv, H.; Wang, Z.-C.; Rao, S.-X.; Zeng, M.-S. Performance comparison between MRI and CT for local staging of sigmoid and descending colon cancer. Eur. J. Radiol. 2019, 121, 108741. [Google Scholar] [CrossRef]
- Yang, L.; Dong, D.; Fang, M.; Zhu, Y.; Zang, Y.; Liu, Z.; Zhang, H.; Ying, J.; Zhao, X.; Tian, J. Can CT-based radiomics signature predict KRAS/NRAS/BRAF mutations in colorectal cancer? Eur. Radiol. 2018, 28, 2058–2067. [Google Scholar] [CrossRef]
- Saadani, H.; Van Der Hiel, B.; Aalbersberg, E.A.; Zavrakidis, I.; Haanen, J.B.; Hoekstra, O.S.; Boellaard, R.; Stokkel, M.P. Metabolic Biomarker–Based BRAFV600 Mutation Association and Prediction in Melanoma. J. Nucl. Med. 2019, 60, 1545–1552. [Google Scholar] [CrossRef]
- Yoon, J.H.; Han, K.; Lee, E.; Lee, J.; Kim, E.-K.; Moon, H.J.; Park, V.Y.; Nam, K.H.; Kwak, J.Y. Radiomics in predicting mutation status for thyroid cancer: A preliminary study using radiomics features for predicting BRAFV600E mutations in papillary thyroid carcinoma. PLoS ONE 2020, 15, e0228968. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mohamed, A.S.; Lai, S.Y.; Yang, S.; Kanwar, A.; Wei, L.; Kamal, M.; Sengupta, S.; Elhalawani, H.; Skinner, H.; et al. Imaging-Genomic Study of Head and Neck Squamous Cell Carcinoma: Associations Between Radiomic Phenotypes and Genomic Mechanisms via Integration of The Cancer Genome Atlas and The Cancer Imaging Archive. JCO Clin. Cancer Inform. 2019, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Elmohr, M.; Fuentes, D.; Habra, M.; Fisher, S.; Perrier, N.; Zhang, M.; Elsayes, K. Radiomic mapping model for prediction of Ki-67 expression in adrenocortical carcinoma. Clin. Radiol. 2020, 75, 479.e17–479.e22. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Ji, Y.; Qi, L.; Guo, X.; Jian, X.; Liu, P. Breast cancer Ki67 expression prediction by DCE-MRI radiomics features. Clin. Radiol. 2018, 73, 909.e1–909.e5. [Google Scholar] [CrossRef]
- Monti, S.; Aiello, M.; Incoronato, M.; Grimaldi, A.M.; Moscarino, M.; Mirabelli, P.; Ferbo, U.; Cavaliere, C.; Salvatore, M. DCE-MRI Pharmacokinetic-Based Phenotyping of Invasive Ductal Carcinoma: A Radiomic Study for Prediction of Histological Outcomes. Contrast Media Mol. Imaging 2018, 2018, 5076269. [Google Scholar] [CrossRef]
- Kierans, A.S.; Rusinek, H.; Lee, A.; Shaikh, M.B.; Triolo, M.; Huang, W.C.; Chandarana, H. Textural Differences in Apparent Diffusion Coefficient Between Low- and High-Stage Clear Cell Renal Cell Carcinoma. Am. J. Roentgenol. 2014, 203, W637–W644. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Xu, L.; Li, Z.; Lv, H.; Dong, N.; Li, W.; Yang, Z.; Wang, Z.; Jin, E. Application of texture analysis based on apparent diffusion coefficient maps in discriminating different stages of rectal cancer. J. Magn. Reson. Imaging 2016, 45, 1798–1808. [Google Scholar] [CrossRef]
- Yin, J.-D.; Song, L.-R.; Lu, H.-C.; Zheng, X. Prediction of different stages of rectal cancer: Texture analysis based on diffusion-weighted images and apparent diffusion coefficient maps. World J. Gastroenterol. 2020, 26, 2082–2096. [Google Scholar] [CrossRef]
- Thompson, H.M.; Bates, D.D.B.; Pernicka, J.G.; Park, S.J.; Nourbakhsh, M.; Fuqua, J.L.; Fiasconaro, M.; Lavery, J.A.; Wei, I.H.; Pappou, E.P.; et al. MRI Assessment of Extramural Venous Invasion before and after Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer and Its Association with Disease-Free and Overall Survival. Ann. Surg. Oncol. 2023, 30, 3957–3965. [Google Scholar] [CrossRef]
- Ao, W.; Zhang, X.; Yao, X.; Zhu, X.; Deng, S.; Feng, J. Preoperative prediction of extramural venous invasion in rectal cancer by dynamic contrast-enhanced and diffusion weighted MRI: A preliminary study. BMC Med. Imaging 2022, 22, 78. [Google Scholar] [CrossRef]
- Brown, G.; Radcliffe, A.G.; Newcombe, R.G.; Dallimore, N.S.; Bourne, M.W.; Williams, G.T. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br. J. Surg. 2003, 90, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Campello, V.M.; Martín-Isla, C.; Izquierdo, C.; Guala, A.; Palomares, J.F.R.; Viladés, D.; Descalzo, M.L.; Karakas, M.; Çavuş, E.; Raisi-Estabragh, Z.; et al. Minimising multi-centre radiomics variability through image normalisation: A pilot study. Sci. Rep. 2022, 12, 12532. [Google Scholar] [CrossRef] [PubMed]




| Parameters | Platform A | Platform A | ||
|---|---|---|---|---|
| T2W | DWI | T2W | DWI | |
| Image orientations | Axial | Axial | Axial | Axial |
| Field of view (mm) | 480–500 | 400–370 | 480–500 | 420–380 |
| Matrix | 564 × 260 | 160 × 200 | 564 × 260 | 150 × 180 |
| Slice thickness (mm) | 5 | 10 | 5 | 10 |
| ETL or FA | Min | 90 FA | Min | 90 FA |
| TR (ms) | 4000 | 8100 | 4200 | 8300 |
| TE (ms) | 85 | 67 | 95 | 73 |
| b values (s/mm2) | 0–1000–1500 | 0–800–1200 | ||
| Number of Averaging (NSA) | 1 | 1 | 1 | 1 |
| Overall | KRAS-Wild | KRAS-Mut | p Value | |
|---|---|---|---|---|
| Patients | 60 | 32 | 28 | |
| Sex (%) | ||||
| male | 35 (58.3) | 15 | 20 | 0.0002 |
| female | 25 (41.7) | 13 | 12 | >0.9999 |
| Age | 57.3 ± 12.9 | 60.2 ± 19.3 | 56.5 ± 16.7 | 0.1392 |
| Weight (kg) | 75.6 ± 17.6 | 71.5 ± 17.7 | 79.8 ± 16.5 | 0.0934 |
| Tumor volume (cm3) | 11.8 ± 16.1 | 19.7 ± 8.8 | 14.6 ± 22.4 | 0.1363 |
| cT stage (%) | ||||
| cT1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| cT2 | 7 (13.5) | 5 (71.5) | 2 (28.5) | 0.8666 |
| cT3 | 22 (42.3) | 14 (63.6) | 8 (36.4) | 0.0197 |
| cT4 | 23 (44.2) | 13 (56.5) | 10 (43.5) | 0.8666 |
| cN stage (%) | ||||
| cN0 | 8 (13.8) | 4 (50) | 4 (50) | >0.9999 |
| cN1 | 8 (13.8) | 2 (25) | 6 (75) | 0.4245 |
| cN2 | 42 (72.4) | 26 (61.9) | 16 (38.1) | <0.0001 |
| cN3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| EMV1 | ||||
| Negative | 18 (25.7) | 11 (61.1) | 7 (38.9) | 0.4245 |
| Positive | 28 (54.3) | 21 (56.3) | 17 (43.7) | 0.4245 |
| Unknown | 14 (20) | 6 (42.9) | 8 (57.1) | 0.8710 |
| MRF | ||||
| Negative | 34 (56.7) | 14 (41.2) | 20 (59.8) | 0.0197 |
| Positive | 22 (36.7) | 10 (45.5) | 12 (54.5) | 0.9977 |
| Unknown | 4 (6.7) | 3 (75) | 1 (25) | 0.6321 |
| Category | ML Model | Training ACCURACY% | Training AUC, CI | Testing ACCURACY% | Testing AUC, CI |
|---|---|---|---|---|---|
| KRAS+/− class | Neural network | 72.93 ± 4.24 | 0.71 ± 0.08 | 62.48 ± 8 * | 0.68 ± 0.25 |
| TNM stage | SVM | 77.6 ± 1.26 | 0.67 ± 0.062 | 72 ± 0.02 | 0.69 ± 0.018 † |
| MRF (positive/negative) | KNN | 62.9 ± 1.75 | 0.55 ± 0.03 | 65.83 ± 0.10 | 0.578 ± 0.055 |
| EMVI (positive/negative) | Logistic regression | 73.1 ± 3.33 | 0.62 ± 0.05 | 76 ± 1.75 | 0.595 ± 0.063 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshuhri, M.S.; Alduhyyim, A.; Al-Mubarak, H.; Alhulail, A.A.; Alomair, O.I.; Madkhali, Y.; Alghuraybi, R.A.; Alotaibi, A.M.; Alqahtani, A.G.M. Investigating the Feasibility of Predicting KRAS Status, Tumor Staging, and Extramural Venous Invasion in Colorectal Cancer Using Inter-Platform Magnetic Resonance Imaging Radiomic Features. Diagnostics 2023, 13, 3541. https://doi.org/10.3390/diagnostics13233541
Alshuhri MS, Alduhyyim A, Al-Mubarak H, Alhulail AA, Alomair OI, Madkhali Y, Alghuraybi RA, Alotaibi AM, Alqahtani AGM. Investigating the Feasibility of Predicting KRAS Status, Tumor Staging, and Extramural Venous Invasion in Colorectal Cancer Using Inter-Platform Magnetic Resonance Imaging Radiomic Features. Diagnostics. 2023; 13(23):3541. https://doi.org/10.3390/diagnostics13233541
Chicago/Turabian StyleAlshuhri, Mohammed S., Abdulaziz Alduhyyim, Haitham Al-Mubarak, Ahmad A. Alhulail, Othman I. Alomair, Yahia Madkhali, Rakan A. Alghuraybi, Abdullah M. Alotaibi, and Abdullalh G. M. Alqahtani. 2023. "Investigating the Feasibility of Predicting KRAS Status, Tumor Staging, and Extramural Venous Invasion in Colorectal Cancer Using Inter-Platform Magnetic Resonance Imaging Radiomic Features" Diagnostics 13, no. 23: 3541. https://doi.org/10.3390/diagnostics13233541