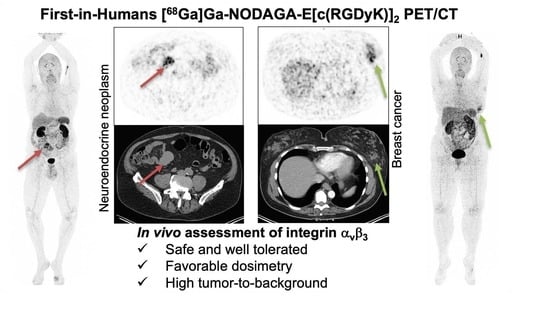
First-in-Human Study of [68Ga]Ga-NODAGA-E[c(RGDyK)]2 PET for Integrin αvβ3 Imaging in Patients with Breast Cancer and Neuroendocrine Neoplasms: Safety, Dosimetry and Tumor Imaging Ability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Synthesis of [68Ga]Ga-NODAGA-E[c(RGDyK)]2
2.3. Plasma Pharmacokinetics and Urine Metabolite Analysis
2.4. PET/CT Acquisition and Image Analysis
2.5. Tumor Uptake by Visual Image Analysis and Activity Quantification
2.6. Dosimetry
2.7. Histology
2.8. Statistics
3. Results
3.1. Patient Characteristics
3.2. Radiochemistry
3.3. Patient Safety and Dosimetry
3.4. Biodistribution and Pharmacokinetics
3.5. Tumor Uptake of [68Ga]Ga-NODAGA-E[c(RGDyK)]2 and Target Validation
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ludwig, B.S.; Kessler, H.; Kossatz, S.; Reuning, U. RGD-Binding Integrins Revisited: How Recently Discovered Functions and Novel Synthetic Ligands (Re-)Shape an Ever-Evolving Field. Cancers 2021, 13, 1711. [Google Scholar] [CrossRef] [PubMed]
- Nieberler, M.; Reuning, U.; Reichart, F.; Notni, J.; Wester, H.J.; Schwaiger, M.; Weinmuller, M.; Rader, A.; Steiger, K.; Kessler, H. Exploring the Role of RGD-Recognizing Integrins in Cancer. Cancers 2017, 9, 116. [Google Scholar] [CrossRef]
- Nabors, L.B.; Mikkelsen, T.; Hegi, M.E.; Ye, X.; Batchelor, T.; Lesser, G.; Peereboom, D.; Rosenfeld, M.R.; Olsen, J.; Brem, S.; et al. A safety run-in and randomized phase 2 study of cilengitide combined with chemoradiation for newly diagnosed glioblastoma (NABTT 0306). Cancer 2012, 118, 5601–5607. [Google Scholar] [CrossRef]
- Reardon, D.A.; Fink, K.L.; Mikkelsen, T.; Cloughesy, T.F.; O’Neill, A.; Plotkin, S.; Glantz, M.; Ravin, P.; Raizer, J.J.; Rich, K.M.; et al. Randomized Phase II Study of Cilengitide, an Integrin-Targeting Arginine-Glycine-Aspartic Acid Peptide, in Recurrent Glioblastoma Multiforme. J. Clin. Oncol. 2008, 26, 5610–5617. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Gorlia, T.; Erridge, S.C.; Perry, J.; Hong, Y.-K.; Aldape, K.D.; Lhermitte, B.; Pietsch, T.; Grujicic, D.; et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1100–1108. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Fukase, Y.; Shang, Y.; Zou, W.; Muñoz-Félix, J.M.; Buitrago, L.; van Agthoven, J.; Zhang, Y.; Hara, R.; Tanaka, Y.; et al. Novel Pure αVβ3 Integrin Antagonists That Do Not Induce Receptor Extension, Prime the Receptor, or Enhance Angiogenesis at Low Concentrations. ACS Pharmacol. Transl. Sci. 2019, 2, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Liolios, C.; Sachpekidis, C.; Kolocouris, A.; Dimitrakopoulou-Strauss, A.; Bouziotis, P. PET Diagnostic Molecules Utilizing Multimeric Cyclic RGD Peptide Analogs for Imaging Integrin αvβ3 Receptors. Molecules 2021, 26, 1792. [Google Scholar] [CrossRef] [PubMed]
- Oxboel, J.; Brandt-Larsen, M.; Madsen, J.; Kjaer, A. Uptake of the Angiogenesis PET Tracer 68Ga-NODAGA-E[c(RGDyK)]2 Correlates Strongly with Angiopoietin-1 and Angiopoietin-2 Expression in Human Neuroendocrine Xenograft Tumors in Mice. J. Nucl. Med. 2016, 57, 1367. [Google Scholar]
- Oxboel, J.; Brandt-Larsen, M.; Schjoeth-Eskesen, C.; Myschetzky, R.; El-Ali, H.H.; Madsen, J.; Kjaer, A. Comparison of two new angiogenesis PET tracers 68Ga-NODAGA-E[c(RGDyK)]2 and 64Cu-NODAGA-E[c(RGDyK)]2; in vivo imaging studies in human xenograft tumors. Nucl. Med. Biol. 2014, 41, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Brandt-Larsen, M.; Oxboel, J.; Kjaer, A.; Madsen, J. Synthesis and evaluation of 68Ga-NODAGA-E[c(RGDyK)]2. J. Label. Compd. Radiopharm. 2013, 56, S205. [Google Scholar]
- Oxboel, J.; Schjoeth-Eskesen, C.; Madsen, J.; El Ali, H.; Kjaer, A. Strong correlation between 64Cu-NODAGA-RGD uptake and quantitative gene expression of integrin-αVβ3 in human neuroendocrine tumor xenografts in mice. J. Nucl. Med. 2012, 53, 1101. [Google Scholar]
- Oxboel, J.; Schjoeth-Eskesen, C.; El-Ali, H.H.; Madsen, J.; Kjaer, A. 64Cu-NODAGA-c(RGDyK) Is a Promising New Angiogenesis PET Tracer: Correlation between Tumor Uptake and Integrin αVβ3 Expression in Human Neuroendocrine Tumor Xenografts. Int. J. Mol. Imaging 2012, 2012, 379807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemmensen, A.; Hansen, A.E.; Holst, P.; Schøier, C.; Bisgaard, S.; Johannesen, H.H.; Ardenkjær-Larsen, J.H.; Kristensen, A.T.; Kjaer, A. [68Ga]Ga-NODAGA-E[(cRGDyK)]2 PET and hyperpolarized [1-13C] pyruvate MRSI (hyperPET) in canine cancer patients: Simultaneous imaging of angiogenesis and the Warburg effect. Eur. J. Nucl. Med. Mol. Imaging 2020, 48, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Bentsen, S.; Clemmensen, A.; Loft, M.; Flethoj, M.; Debes, K.P.; Ludvigsen, T.P.; Larsen, C.B.; Kirchhoff, J.; Olsen, L.H.; Moller, J.E.; et al. [68Ga]Ga-NODAGA-E[(cRGDyK)]2 Angiogenesis PET/MR in a Porcine Model of Chronic Myocardial Infarction. Diagnostics 2021, 11, 1807. [Google Scholar] [CrossRef]
- Hansen, H.D.; Ettrup, A.; Herth, M.M.; Dyssegaard, A.; Ratner, C.; Gillings, N.; Knudsen, G.M. Direct comparison of [18F]MH.MZ and [18F] altanserin for 5-HT2A receptor imaging with PET. Synapse 2013, 67, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Gillings, N. A restricted access material for rapid analysis of [11C]-labeled radiopharmaceuticals and their metabolites in plasma. Nucl. Med. Biol. 2009, 36, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Stabin, M.G.; Siegel, J.A. Physical models and dose factors for use in internal dose assessment. Health Phys. 2003, 85, 294–310. [Google Scholar] [CrossRef]
- Stabin, M.G.; Siegel, J.A. RADAR Dose Estimate Report: A Compendium of Radiopharmaceutical Dose Estimates Based on OLINDA/EXM Version 2.0. J. Nucl. Med. 2018, 59, 154–160. [Google Scholar] [CrossRef]
- Goodman, S.L.; Grote, H.J.; Wilm, C. Matched rabbit monoclonal antibodies against alphav-series integrins reveal a novel αvβ3-LIBS epitope, and permit routine staining of archival paraffin samples of human tumors. Biol. Open 2012, 1, 329–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The 2007 Recommendations of the International Commission on Radiological Protection; ICRP Publication: Stockholm, Switzerland, 2007; Volume 37. [CrossRef]
- Quinn, B.; Dauer, Z.; Pandit-Taskar, N.; Schoder, H.; Dauer, L.T. Radiation dosimetry of 18F-FDG PET/CT: Incorporating exam-specific parameters in dose estimates. BMC Med. Imaging 2016, 16, 41. [Google Scholar] [CrossRef]
- Chen, H.; Niu, G.; Wu, H.; Chen, X. Clinical Application of Radiolabeled RGD Peptides for PET Imaging of Integrin αvβ3. Theranostics 2016, 6, 78–92. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Wang, S.; Zhang, X.; Teng, Z.; Wang, J.; Yung, B.C.; Niu, G.; Zhu, H.; Lu, G.; Chen, X. 18F-Alfatide II PET/CT for Identification of Breast Cancer: A Preliminary Clinical Study. J. Nucl. Med. 2018, 59, 1809–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Wang, H.; Tan, H.; Cui, X.; Yao, S.; Zang, J.; Zhang, L.; Zhu, Z. Evaluation of Lung Cancer and Neuroendocrine Neoplasm in a Single Scan by Targeting Both Somatostatin Receptor and Integrin αvβ3. Clin. Nucl. Med. 2019, 44, 687–694. [Google Scholar] [CrossRef]
- Durante, S.; Dunet, V.; Gorostidi, F.; Mitsakis, P.; Schaefer, N.; Delage, J.; Prior, J.O. Head and neck tumors angiogenesis imaging with 68Ga-NODAGA-RGD in comparison to 18F-FDG PET/CT: A pilot study. EJNMMI Res. 2020, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Gnesin, S.; Mitsakis, P.; Cicone, F.; Deshayes, E.; Dunet, V.; Gallino, A.F.; Kosinski, M.; Baechler, S.; Buchegger, F.; Viertl, D.; et al. First in-human radiation dosimetry of 68Ga-NODAGA-RGDyK. EJNMMI Res. 2017, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Minamimoto, R.; Karam, A.; Jamali, M.; Barkhodari, A.; Gambhir, S.S.; Dorigo, O.; Iagaru, A. Pilot prospective evaluation of 18F-FPPRGD2 PET/CT in patients with cervical and ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1047–1055. [Google Scholar] [CrossRef] [PubMed]






| Patient No. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Sex | Male | Male | Female | Female | Male | Female | Female | Female | Female | Male |
| Age (y) | 69 | 79 | 55 | 67 | 58 | 58 | 68 | 68 | 63 | 52 |
| Cancer type | Neuroendocrine | Neuroendocrine | Neuroendocrine | Breast | Neuroendocrine | Breast | Breast | Breast | Breast | Neuroendocrine |
| Stage/grade | PT in small intestine, metastases in the liver and mesentery | PT in small intestine, metastasis in the mesentery | PT in terminal ileum/coecum | PT left breast | PT not identified, liver metastasis | PT in right breast, SN without metastases | PT in right breast, metastases in 3/14 LN, no distant metastases | PT in left breast, SN without metastases | PT in left breast, SN without metastases | PT in pancreas, liver-, bone, and lymph node metastases |
| Biomarker status | Ki67 2% * | Ki67 2% | Ki67 1% | ER 100%, HER2 borderline | Ki67 14% | ER 100%, HER2 neg. | ER neg., HER2 neg. | ER 100%, HER2 neg. | ER 100%, HER2 neg. | Ki67 25% * |
| Concurrent cancer treatment | Lanreotid | Lanreotid | Lanreotid | None | None | None | None | None | None | None |
| Days from PET scan to biopsy/operation | 6 | 14 | 30 | 5 | 18 | 6 | 1 | 5 | 5 | NA |
| Tissue | NA | Fresh frozen, later paraffin embedded | Fresh frozen, later paraffin embedded | Paraffin embedded | Paraffin embedded | Paraffin embedded | Paraffin embedded | Paraffin embedded | Paraffin embedded | NA |
| Organ/Tissue | Mean Absorbed Dose (mGy/MBq) |
|---|---|
| Adrenals | 0.02450 |
| Brain | 0.00252 |
| Breasts | 0.01050 |
| Esophagus | 0.01120 |
| Eyes | 0.00929 |
| Gallbladder Wall | 0.01430 |
| Left Colon | 0.01360 |
| Small Intestine | 0.06030 |
| Stomach Wall | 0.02630 |
| Right Colon | 0.01310 |
| Rectum | 0.01510 |
| Heart Wall | 0.01460 |
| Kidneys | 0.06270 |
| Liver | 0.02790 |
| Lungs | 0.00792 |
| Ovaries | 0.01540 |
| Pancreas | 0.01440 |
| Prostate | 0.01330 |
| Salivary Glands | 0.00996 |
| Red Marrow | 0.01500 |
| Osteogenic Cells | 0.01360 |
| Spleen | 0.05040 |
| Testes | 0.01920 |
| Thymus | 0.01100 |
| Thyroid | 0.06630 |
| Urinary Bladder Wall | 0.12600 |
| Uterus | 0.01800 |
| Total Body | 0.01330 |
| Effective Dose (mSv/MBq) | 0.02180 |
| Patient No. | Tumor Type | Tumor Size | Qualitative PET Uptake | SUVmax | SUVmean | ||||
|---|---|---|---|---|---|---|---|---|---|
| PET10 | PET1h | PET2h | PET10 | PET1h | PET2h | ||||
| 1 | NEN | 4.4 cm | Heterogeneous | 4.53 | 4.55 | 5.70 | 2.58 | 2.37 | 2.93 |
| 2 | NEN | 4.9 cm | Heterogeneous | 10.36 | 17.70 | 14.32 | 5.31 | 8.74 | 7.73 |
| 3 | NEN | 4.4 cm | Heterogeneous | 7.85 | 8.77 | 15.35 | 4.10 | 4.48 | 7.86 |
| 4 | BC | 6 cm | Heterogeneous | 6.18 | 8.75 | 10.53 | 3.26 | 4.52 | 5.44 |
| 5 | NEN | 16 cm * | Heterogeneous | 7.39 | 9.39 | 8.83 | 2.93 | 3.2 | 3.15 |
| 6 | BC | 1.1 cm | Homogeneous | 4.88 | 7.15 | 8.02 | 4.59 | 6.75 | 6.79 |
| 7 | BC | 1.4 cm | Homogeneous | 3.05 | 2.29 | 2.66 | 1.67 | 1.30 | 1.94 |
| 8 | BC | 1.8 cm | Homogeneous | 7.09 | 8.40 | 7.10 | 4.18 | 4.70 | 4.04 |
| 9 | BC | 0.9 cm | Homogeneous | 4.24 | 4.99 | 4.45 | 2.36 | 2.54 | 2.58 |
| 10 | NEN | 10 cm | Heterogeneous | 5.90 | 7.58 | 5.80 | 3.27 | 3.69 | 3.05 |
| PET 10 min p.i. | PET 1 h p.i. | PET 2 h p.i. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BC | NEN | All | BC | NEN | All | BC | NEN | All | |
| Tumor to blood | 2.79 (0.45) | 3.96 (0.61) | 3.37 (0.40) | 5.72 (1.27) | 9.40 (2.06) | 7.56 (1.30) | 12.1 (4.11) | 11.4 (1.81) | 11.7 (2.12) |
| Tumor to liver | 2.37 (0.43) | 2.58 (0.30) | 2.48 (0.25) | 3.18 (0.67) | 3.21 (0.69) | 3.20 (0.45) | 2.67 (0.55) | 2.89 (0.36) | 2.78 (0.31) |
| Tumor to kidney | 0.60 (0.14) | 0.81 (0.16) | 0.70 (0.11) | 1.07 (0.24) | 1.48 (0.26) | 1.27 (0.18) | 1.08 (0.23) | 1.56 (0.24) | 1.32 (0.18) |
| Tumor to muscle | 7.11 (1.42) | 10.2 (1.81) | 8.64 (1.20) | 11.9 (4.36) | 15.5 (4.08) | 13.7 (2.88) | 7.40 (2.68) | 11.4 (3.35) | 9.42 (2.13) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clausen, M.M.; Carlsen, E.A.; Christensen, C.; Madsen, J.; Brandt-Larsen, M.; Klausen, T.L.; Holm, S.; Loft, A.; Berthelsen, A.K.; Kroman, N.; et al. First-in-Human Study of [68Ga]Ga-NODAGA-E[c(RGDyK)]2 PET for Integrin αvβ3 Imaging in Patients with Breast Cancer and Neuroendocrine Neoplasms: Safety, Dosimetry and Tumor Imaging Ability. Diagnostics 2022, 12, 851. https://doi.org/10.3390/diagnostics12040851
Clausen MM, Carlsen EA, Christensen C, Madsen J, Brandt-Larsen M, Klausen TL, Holm S, Loft A, Berthelsen AK, Kroman N, et al. First-in-Human Study of [68Ga]Ga-NODAGA-E[c(RGDyK)]2 PET for Integrin αvβ3 Imaging in Patients with Breast Cancer and Neuroendocrine Neoplasms: Safety, Dosimetry and Tumor Imaging Ability. Diagnostics. 2022; 12(4):851. https://doi.org/10.3390/diagnostics12040851
Chicago/Turabian StyleClausen, Malene Martini, Esben Andreas Carlsen, Camilla Christensen, Jacob Madsen, Malene Brandt-Larsen, Thomas Levin Klausen, Søren Holm, Annika Loft, Anne Kiil Berthelsen, Niels Kroman, and et al. 2022. "First-in-Human Study of [68Ga]Ga-NODAGA-E[c(RGDyK)]2 PET for Integrin αvβ3 Imaging in Patients with Breast Cancer and Neuroendocrine Neoplasms: Safety, Dosimetry and Tumor Imaging Ability" Diagnostics 12, no. 4: 851. https://doi.org/10.3390/diagnostics12040851