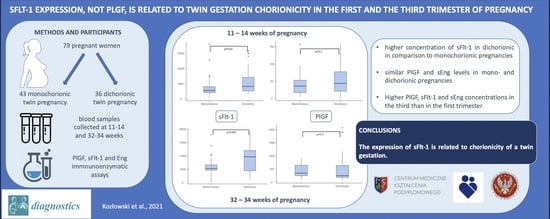
sFlt-1, Not PlGF, Is Related to Twin Gestation Choronicity in the First and Third Trimesters of Pregnancy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef] [Green Version]
- Fox, R.; Kitt, J.; Leeson, P.; Aye, C.Y.L.; Lewandowski, A.J. Preeclampsia: Risk Factors, Diagnosis, Management, and the Cardiovascular Impact on the Offspring. J. Clin. Med. 2019, 8, 1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redman, C.W.; Staff, A.C. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am. J. Obstet. Gynecol. 2015, 213, S9–S11. [Google Scholar] [CrossRef]
- Yagel, S.; Cohen, S.M.; Goldman-Wohl, D. An integrated model of preeclampsia: A multifaceted syndrome of the maternal cardiovascular-placental-fetal array. Am. J. Obstet. Gynecol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Birdir, C.; Droste, L.; Fox, L.; Frank, M.; Fryze, J.; Enekwe, A.; Köninger, A.; Kimmig, R.; Schmidt, B.; Gellhaus, A. Predictive value of sFlt-1, PlGF, sFlt-1/PlGF ratio and PAPP-A for late-onset preeclampsia and IUGR between 32 and 37 weeks of pregnancy. Pregnancy Hypertens. 2018, 12, 124–128. [Google Scholar] [CrossRef]
- Venkatesha, S.; Toporsian, M.; Lam, C.; Hanai, J.; Mammoto, T.; Kim, Y.M.; Bdolah, Y.; Lim, K.H.; Yuan, H.T.; Libermann, T.A.; et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006, 12, 642–649. [Google Scholar] [CrossRef]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, O.; Llurba, E.; Marsal, G.; Domínguez, C.; Aulesa, C.; Sánchez-Durán, M.A.; Goya, M.M.; Alijotas-Reig, J.; Carreras, E.; Cabero, L. First trimester serum angiogenic/anti-angiogenic status in twin pregnancies: Relationship with assisted reproduction technology. Hum. Reprod. 2012, 27, 358–365. [Google Scholar] [CrossRef]
- Svirsky, R.; Levinsohn-Tavor, O.; Feldman, N.; Klog, E.; Cuckle, H.; Maymon, R. First- and second-trimester maternal serum markers of pre-eclampsia in twin pregnancy. Ultrasound Obstet. Gynecol. 2016, 47, 560–564. [Google Scholar] [CrossRef] [Green Version]
- Narang, K.; Szymanski, L.M. Multiple Gestations and Hypertensive Disorders of Pregnancy: What Do We Know? Curr. Hypertens. Rep. 2020, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Kosinska-Kaczynska, K.; Zgliczynska, M.; Kozlowski, S.; Wicherek, L. Maternal Serum Placental Growth Factor, Soluble Fms-Like Tyrosine Kinase-1, and Soluble Endoglin in Twin Gestations and the Risk of Preeclampsia—A Systematic Review. J. Clin. Med. 2020, 9, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laine, K.; Murzakanova, G.; Sole, K.B.; Pay, A.D.; Heradstveit, S.; Räisänen, S. Prevalence and risk of pre-eclampsia and gestational hypertension in twin pregnancies: A population-based register study. BMJ Open 2019, 9, e029908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartnik, P.; Kosinska-Kaczynska, K.; Kacperczyk, J.; Ananicz, W.; Sierocińska, A.; Wielgos, M.; Szymusik, I. Twin Chorionicity and the Risk of Hypertensive Disorders: Gestational Hypertension and Pre-eclampsia. Twin Res. Hum. Genet. 2016, 19, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Sarno, L.; Maruotti, G.M.; Donadono, V.; Saccone, G.; Martinelli, P. Risk of preeclampsia: Comparison between dichorionic and monochorionic twin pregnancies. J. Matern. Fetal Neonatal Med. 2014, 27, 1080–1081. [Google Scholar] [CrossRef] [Green Version]
- Sparks, T.N.; Cheng, Y.W.; Phan, N.; Caughey, A.B. Does risk of preeclampsia differ by twin chorionicity? J. Matern. Fetal Neonatal Med. 2013, 26, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Francisco, C.; Wright, D.; Benkő, Z.; Syngelaki, A.; Nicolaides, K.H. Competing-risks model in screening for pre-eclampsia in twin pregnancy according to maternal factors and biomarkers at 11–13 weeks’ gestation. Ultrasound Obstet. Gynecol. 2017, 50, 589–595. [Google Scholar] [CrossRef]
- ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2019, 133, 1.
- Wender-Ożegowska, E.; Bomba-Opoń, D.; Brązert, J.; Celewicz, Z.; Czajkowski, K.; Gutaj, P.; Malinowska-Polubiec, A.; Zawiejska, A.; Wielgoś, M. Standards of Polish Society of Gynecologists and Obstetricians in management of women with diabetes. Ginekol. Pol. 2018, 89, 341–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maynard, S.E.; Moore Simas, T.A.; Solitro, M.J.; Rajan, A.; Crawford, S.; Soderland, P.; Meyer, B.A. Circulating angiogenic factors in singleton vs multiple-gestation pregnancies. Am. J. Obstet. Gynecol. 2008, 198, 200.e1–200.e7. [Google Scholar] [CrossRef]
- Ruiz-Sacedón, N.; Perales-Puchalt, A.; Borras, D.; Gómez, R.; Perales, A. Angiogenic growth factors in maternal and fetal serum in concordant and discordant twin pregnancies. J. Matern. Fetal Neonatal Med. 2014, 27, 870–873. [Google Scholar] [CrossRef]
- Faupel-Badger, J.M.; McElrath, T.F.; Lauria, M.; Houghton, L.C.; Lim, K.H.; Parry, S.; Cantonwine, D.; Lai, G.; Karumanchi, S.A.; Hoover, R.N.; et al. Maternal circulating angiogenic factors in twin and singleton pregnancies. Am. J. Obstet. Gynecol. 2015, 212, 636.e1–636.e8. [Google Scholar] [CrossRef] [Green Version]
- Cowans, N.J.; Spencer, K. First trimester maternal serum placental growth factor levels in twin pregnancies. Prenat. Diagn. 2013, 33, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Bdolah, Y.; Lam, C.; Rajakumar, A.; Shivalingappa, V.; Mutter, W.; Sachs, B.P.; Lim, K.H.; Bdolah-Abram, T.; Epstein, F.H.; Karumanchi, S.A. Twin pregnancy and the risk of preeclampsia: Bigger placenta or relative ischemia? Am. J. Obstet. Gynecol. 2008, 198, 428.e1–428.e6. [Google Scholar] [CrossRef] [PubMed]
- Wathén, K.A.; Tuutti, E.; Stenman, U.H.; Alfthan, H.; Halmesmäki, E.; Finne, P.; Ylikorkala, O.; Vuorela, P. Maternal serum-soluble vascular endothelial growth factor receptor-1 in early pregnancy ending in preeclampsia or intrauterine growth retardation. J. Clin. Endocrinol. Metab. 2006, 91, 180–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagamatsu, T.; Fujii, T.; Kusumi, M.; Zou, L.; Yamashita, T.; Osuga, Y.; Momoeda, M.; Kozuma, S.; Taketani, Y. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: An implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 2004, 145, 4838–4845. [Google Scholar] [CrossRef] [PubMed]
- Saleh, L.; Tahitu, S.I.M.; Danser, A.H.J.; van den Meiracker, A.H.; Visser, W. The predictive value of the sFlt-1/PlGF ratio on short-term absence of preeclampsia and maternal and fetal or neonatal complications in twin pregnancies. Pregnancy Hypertens. 2018, 14, 222–227. [Google Scholar] [CrossRef]
- Boucoiran, I.; Thissier-Levy, S.; Wu, Y.; Wei, S.Q.; Luo, Z.C.; Delvin, E.; Fraser, W.D.; Audibert, F.; MIROS Study Group. Risks for preeclampsia and small for gestational age: Predictive values of placental growth factor, soluble fms-like tyrosine kinase-1, and inhibin A in singleton and multiple-gestation pregnancies. Am. J. Perinatol. 2013, 30, 607–612. [Google Scholar] [PubMed]


| Study Group N = 79 | Monochorionic Pregnancies N = 43 | Dichorionic Pregnancies N = 36 | p | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Age (years) * | 31.5 ± 4.3 | 31.8 ± 4.3 | 31.3 ± 4.3 | 0.7 |
| Primiparity | 39 (49.4) | 23 (53.5) | 16 (44.4) | 0.3 |
| Smoking status | 0.8 | |||
| current | 3 (3.8) | 2 (4.7) | 1 (2.8) | |
| past | 45 (57) | 23 (53.4) | 22 (61.1) | |
| never | 28 (35.4) | 16 (37.2) | 12 (33.3) | |
| unknown | 3 (3.8) | 2 (4.7) | 1 (2.8) | |
| Pre-gravid BMI (kg/m2) * | 23.8 ± 4.6 | 23.1 ± 4.6 | 24.6 ± 4.6 | 0.08 |
| Obesity | 8 (10.1) | 4 (9.3) | 4 (11.1) | 0.4 |
| Gestational weight gain * | 18.7 ± 8.6 | 17.8 ± 8.9 | 19.6 ± 8.4 | 0.3 |
| ART | 17 (21.5) | 7 (16.3) | 10 (27.8) | 0.3 |
| Gestational hypertension | 9 (11.4) | 4 (9.3) | 5 (13.9) | 0.7 |
| Early PE | 2 (2.5) | 0 | 2 (5.6) | 0.3 |
| Late PE | 1 (1.3) | 0 | 1 (2.8) | 0.8 |
| GDM | 21 (26.6) | 11 (25.6) | 10 (27.8) | 0.5 |
| Gestational age at delivery (weeks) * | 35.7 ± 1.9 | 35.5 ± 1.7 | 35.9 ± 2.2 | 0.4 |
| Cesarean delivery | 46 (58.2) | 24 (55.8) | 22 (61.1) | 0.8 |
| First twin birthweight (g) * | 2423 ± 465 | 2383 ± 488 | 2472 ± 441 | 0.7 |
| First twin 5th minute Apgar ≤ 7 points | 3 (3.8) | 2 (4.6) | 1 (2.8) | 0.6 |
| Second twin birthweight (g) * | 2360 ± 409 | 2314 ± 420 | 2417 ± 397 | 0.3 |
| Second twin 5th minute Apgar ≤ 7 points | 6 (7.6) | 3 (7) | 3 (8.3) | 0.9 |
| Discordant inter-twin birthweight | 7 (8.9) | 4 (9.3) | 3 (8.3) | 0.4 |
| 1st Trimester | 3rd Trimester | p | 1st Trimester | 3rd Trimester | p | |
|---|---|---|---|---|---|---|
| Median (Interquartile Range) | Median (Interquartile Range) | Median (Interquartile Range) | Median (Interquartile Range) | |||
| Monochorionic | Dichorionic | |||||
| PlGF (pg/mL) | 36.3 (17.8–53.2) | 345 (243–644) | <0.001 | 44.3 (21.5–77.8) | 246.7 (184–635) | <0.001 |
| sFlt-1 (pg/mL) | 2830.9 (2140–4042) | 5306 (4706–6679) | <0.001 | 4156.4 (2668–7099) | 9692 (4489–12004) | <0.001 |
| sFlt-1/PlGF | 99.1 (45.1–160.1) | 14.5 (7.7–26.5) | <0.001 | 108.1 (45.3–288.2) | 37.3 (9.7–52.6) | <0.001 |
| Eng (ng/mL) | 0.1 (0.1–0.4) | 0.36 (0.1–0.9) | 0.046 | 0.17 (0.1–0.4) | 0.62 (0.2–0.8) | 0.001 |
| 1st Trimester | 3rd Trimester | p | 1st Trimester | 3rd Trimester | p | |
|---|---|---|---|---|---|---|
| Median (Interquartile Range) | Median (Interquartile Range) | Median (Interquartile Range) | Median (Interquartile Range) | |||
| Monochorionic | Dichorionic | |||||
| PlGF (pg/mL) | 36.5 (17.8–54.1) | 348 (245–652) | <0.001 | 48.7 (23.5–79.1) | 257 (184–644) | <0.001 |
| sFlt-1 (pg/mL) | 2829.2 (2140–4041) | 5300 (4703–6671) | <0.001 | 4112.1 (2651–7083) | 9653 (4411–11752) | <0.001 |
| sFlt-1/PlGF | 98.2 (45–159.1) | 14.4 (7.5–26.5) | <0.001 | 103.9 (42.8–265.7) | 35.9 (9.2–49.6) | <0.001 |
| Eng (ng/mL) | 0.1 (0.1–0.4) | 0.35 (0.1–0.9) | 0.049 | 0.14 (0.1–0.4) | 0.6 (0.2–0.9) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozłowski, S.; Stelmaszczyk-Emmel, A.; Szymusik, I.; Saletra-Bielińska, A.; Brawura-Biskupski-Samaha, R.; Pietruski, P.; Osińska, A.; Kosińska-Kaczyńska, K. sFlt-1, Not PlGF, Is Related to Twin Gestation Choronicity in the First and Third Trimesters of Pregnancy. Diagnostics 2021, 11, 1181. https://doi.org/10.3390/diagnostics11071181
Kozłowski S, Stelmaszczyk-Emmel A, Szymusik I, Saletra-Bielińska A, Brawura-Biskupski-Samaha R, Pietruski P, Osińska A, Kosińska-Kaczyńska K. sFlt-1, Not PlGF, Is Related to Twin Gestation Choronicity in the First and Third Trimesters of Pregnancy. Diagnostics. 2021; 11(7):1181. https://doi.org/10.3390/diagnostics11071181
Chicago/Turabian StyleKozłowski, Szymon, Anna Stelmaszczyk-Emmel, Iwona Szymusik, Aleksandra Saletra-Bielińska, Robert Brawura-Biskupski-Samaha, Paweł Pietruski, Agnieszka Osińska, and Katarzyna Kosińska-Kaczyńska. 2021. "sFlt-1, Not PlGF, Is Related to Twin Gestation Choronicity in the First and Third Trimesters of Pregnancy" Diagnostics 11, no. 7: 1181. https://doi.org/10.3390/diagnostics11071181