Serum High-Sensitivity C-Reactive Protein Is Associated with Postoperative Psychiatric Status in Patients with Empty Nose Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Enrollment
2.2. Associated Parameters
2.3. Measurements of Nasal–Facial Symptoms and Psychiatric Status
2.4. Surgical Intervention and Postoperative Protocol
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. The Change in Serum hs-CRP Level after Surgery
3.3. Correlation and ROC Curve Analysis for Preoperative hs-CRP
3.4. Regression Analysis for Postoperative Depression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sozansky, J.; Houser, S.M. Pathophysiology of empty nose syndrome. Laryngoscope 2015, 125, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.J.; Kern, E.B. Atrophic rhinitis: A review of 242 cases. Am. J. Rhinol. 2001, 15, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.; Dessi, P.; Serrano, E. Empty nose syndrome. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2012, 129, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Chhabra, N.; Houser, S.M. The diagnosis and management of empty nose syndrome. Otolaryngol. Clin. N. Am. 2009, 42, 311–330. [Google Scholar] [CrossRef]
- Lee, T.J.; Fu, C.H.; Wu, C.L.; Tam, Y.Y.; Huang, C.C.; Chang, P.H.; Chen, Y.W.; Wu, M.H. Evaluation of depression and anxiety in empty nose syndrome after surgical treatment. Laryngoscope 2016, 126, 1284–1289. [Google Scholar] [CrossRef]
- Manji, J.; Nayak, J.V.; Thamboo, A. The functional and psychological burden of empty nose syndrome. Int. Forum Allergy Rhinol. 2018, 8, 707–712. [Google Scholar] [CrossRef]
- Lemogne, C.; Consoli, S.M.; Limosin, F.; Bonfils, P. Treating empty nose syndrome as a somatic symptom disorder. Gen. Hosp. Psychiatry 2015, 37, 273.e9-10. [Google Scholar] [CrossRef] [PubMed]
- Bastier, P.L.; Fierens, S.; Champel, S.; Ribadeau-Dumas, A.; de Gabory, L. β-Tricalcium Phosphate Implants in the Surgical Treatment of Empty Nose Syndrome. Otolaryngol. Head Neck Surg. 2016, 155, 514–517. [Google Scholar] [CrossRef]
- Houser, S.M. Surgical treatment for empty nose syndrome. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 858–863. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.J.; Fu, C.H.; Wu, C.L.; Lee, Y.C.; Huang, C.C.; Chang, P.H.; Chen, Y.W.; Tseng, H.J. Surgical outcome for empty nose syndrome: Impact of implantation site. Laryngoscope 2018, 128, 554–559. [Google Scholar] [CrossRef]
- Ma, Z.X.; Quan, Z.; Jie, L.; Hu, G.H. Assessment of postsurgical outcomes between different implants in patients with empty nose syndrome: A meta-analysis. J. Int. Med. Res. 2017, 45, 1939–1948. [Google Scholar] [CrossRef]
- Houser, S.M. Empty nose syndrome associated with middle turbinate resection. Otolaryngol. Head Neck Surg. 2006, 135, 972–973. [Google Scholar] [CrossRef]
- Tam, Y.Y.; Lee, T.J.; Wu, C.C.; Chang, P.H.; Chen, Y.W.; Fu, C.H.; Huang, C.C. Clinical analysis of submucosal Medpor implantation for empty nose syndrome. Rhinology 2014, 52, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.L.; Fu, C.H.; Lee, T.J. Distinct histopathology characteristics in empty nose syndrome. Laryngoscope 2021, 131, E14–E18. [Google Scholar]
- Kaufman, J.; DeLorenzo, C.; Choudhury, S.; Parsey, R.V. The 5-HT 1A receptor in major depressive disorder. Eur. Neuropsychopharmacol. 2016, 26, 397–410. [Google Scholar] [CrossRef] [Green Version]
- Molendijk, M.; Spinhoven, P.; Polak, M.; Bus, B.; Penninx, B.; Elzinga, B. Serum BDNF concentrations as peripheral manifestations of depression: Evidence from a systematic review and meta-analyses on 179 associations. Mol. Psychiatry 2014, 19, 791–800. [Google Scholar] [CrossRef]
- Ornell, F.; Hansen, F.; Schuch, F.B.; Rebelatto, F.P.; Tavares, A.L.; Scherer, J.N.; Valerio, A.G.; Pechansky, F.; Kessler, F.H.P.; von Diemen, L. Brain-derived neurotrophic factor in substance use disorders: A systematic review and meta-analysis. Drug Alcohol Depend. 2018, 193, 91–103. [Google Scholar] [CrossRef]
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef]
- Tseng, P.T.; Cheng, Y.S.; Chen, Y.W.; Wu, C.K.; Lin, P.Y. Increased levels of vascular endothelial growth factor in patients with major depressive disorder: A meta-analysis. Eur. Neuropsychopharmacol. 2015, 25, 1622–1630. [Google Scholar] [CrossRef]
- Kader, F.; Ghai, M.; Maharaj, L. The effects of DNA methylation on human psychology. Behav. Brain Res. 2018, 346, 47–65. [Google Scholar] [CrossRef]
- Tomasi, J.; Lisoway, A.J.; Zai, C.C.; Harripaul, R.; Müller, D.J.; Zai, G.C.M.; McCabe, R.E.; Richter, M.A.; Kennedy, J.L.; Tiwari, A.K. Towards precision medicine in generalized anxiety disorder: Review of genetics and pharmaco(epi)genetics. J. Psychiatr. Res. 2019, 119, 33–47. [Google Scholar] [CrossRef]
- Schiele, M.A.; Gottschalk, M.G.; Domschke, K. The applied implications of epigenetics in anxiety, affective and stress-related disorders—A review and synthesis on psychosocial stress, psychotherapy and prevention. Clin. Psychol. Rev. 2020, 77, 101830. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; Schratt, G. miRNA regulation of social and anxiety-related behaviour. Cell Mol. Life Sci. 2020, 77, 4347–4364. [Google Scholar] [CrossRef]
- Chen, F.; Zou, L.; Dai, Y.; Sun, J.; Chen, C.; Zhang, Y.; Peng, Q.; Zhang, Z.; Xie, Z.; Wu, H.; et al. Prognostic plasma exosomal microRNA biomarkers in patients with substance use disorders presenting comorbid with anxiety and depression. Sci. Rep. 2021, 11, 6271. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Tansey, K.E.; Schalkwyk, L.C.; Powell, T.R. The inflammatory cytokines: Molecular biomarkers for major depressive disorder? Biomark. Med. 2015, 9, 169–180. [Google Scholar] [CrossRef]
- Felger, J.C. Imaging the role of inflammation in mood and anxiety-related disorders. Curr. Neuropharmacol. 2018, 16, 533–558. [Google Scholar] [CrossRef]
- Milenkovic, V.M.; Stanton, E.H.; Nothdurfter, C.; Rupprecht, R.; Wetzel, C.H. The role of chemokines in the pathophysiology of major depressive disorder. Int. J. Mol. Sci. 2019, 20, 2283. [Google Scholar] [CrossRef] [Green Version]
- Wium-Andersen, M.K.; Ørsted, D.D.; Nielsen, S.F.; Nordestgaard, B.G. Elevated C-reactive protein levels, psychological distress, and depression in 73,131 individuals. JAMA Psychiatry 2013, 70, 176–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maes, M.; Kubera, M.; Obuchowiczwa, E.; Goehler, L.; Brzeszcz, J. Depression’s multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways. Neuroendocrinol. Lett. 2011, 32, 7–24. [Google Scholar]
- Valkanova, V.; Ebmeier, K.P.; Allan, C.L. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 2013, 150, 736–744. [Google Scholar] [CrossRef]
- Cepeda, M.S.; Stang, P.; Makadia, R. Depression is associated with high levels of C-reactive protein and low levels of fractional exhaled nitric oxide: Results from the 2007–2012 national health and nutrition examination surveys. J. Clin. Psychiatry 2016, 77, 1666–1671. [Google Scholar] [CrossRef]
- Zainal, N.H.; Newman, M.G. Increased inflammation predicts nine-year change in major depressive disorder diagnostic status. J. Abnorm. Psychol. 2021, 130, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Pasco, J.A.; Nicholson, G.C.; Williams, L.J.; Jacka, F.N.; Henry, M.J.; Kotowicz, M.A.; Schneider, H.G.; Leonard, B.E.; Merk, M. Association of high-sensitivity C-reactive protein with de novo major depression. Br. J. Psychiatry 2010, 197, 372–377. [Google Scholar] [CrossRef] [Green Version]
- Hunt, C.; Cordeiro, T.M.E.; Suchting, R.; de Dios, C.; Leal, V.A.C.; Soares, J.C.; Dantzer, R.; Teixeira, A.L.; Selvaraj, S. Effect of immune activation on the kynurenine pathway and depression symptoms—A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020, 118, 514–523. [Google Scholar] [CrossRef]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune influencers in action: Metabolites and enzymes of the tryptophan-kynurenine metabolic pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Saccaro, L.F.; Schilliger, Z.; Perroud, N.; Piguet, C. Inflammation, anxiety, and stress in attention-deficit/hyperactivity disorder. Biomedicines 2021, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, N.; Thamboo, A.; Habib, A.R.; Huang, Z.; Nayak, J.V. The Empty Nose Syndrome 6-Item Questionnaire (ENS6Q): A validated 6-item questionnaire as a diagnostic aid for empty nose syndrome patients. Int. Forum Allergy Rhinol. 2017, 7, 64–71. [Google Scholar] [CrossRef]
- Ledue, T.B.; Weiner, D.L.; Sipe, J.D.; Poulin, S.E.; Collins, M.F.; Rifai, N. Analytical evaluation of particle-enhanced immunonephelometric assays for C-reactive protein, serum amyloid A and mannose-binding protein in human serum. Ann. Clin. Biochem. 1998, 35, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Brown, G.K. BDI-II, Beck Depression Inventory: Manual, 2nd ed.; Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef]
- Fu, C.H.; Wu, C.L.; Huang, C.C.; Chang, P.H.; Chen, Y.W.; Lee, T.J. Nasal nitric oxide in relation to psychiatric status of patients with empty nose syndrome. Nitric Oxide 2019, 92, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Bassuk, S.S.; Rifai, N.; Ridker, P.M. High-sensitivity C-reactive protein: Clinical importance. Curr. Probl. Cardiol. 2004, 29, 439–493. [Google Scholar] [PubMed]
- Helal, I.; Zerelli, L.; Krid, M.; ElYounsi, F.; Maiz, H.B.; Zouari, B.; Adelmoula, J.; Kheder, A. Comparison of C-reactive protein and high-sensitivity C-reactive protein levels in patients on hemodialysis. Saudi J. Kidney Dis. Transpl. 2012, 23, 477–483. [Google Scholar]
- Freund, W.; Wunderlich, A.P.; Stocker, T.; Schmitz, B.L.; Scheithauer, M.O. Empty nose syndrome: Limbic system activation observed by functional magnetic resonance imaging. Laryngoscope 2011, 121, 2019–2025. [Google Scholar] [CrossRef]
- McGarry, L.M.; Carter, A.G. Prefrontal Cortex Drives Distinct Projection Neurons in the Basolateral Amygdala. Cell Rep. 2017, 21, 1426–1433. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, C.W. Role of amygdala-prefrontal cortex circuitry in regulating the expression of contextual fear memory. Neurobiol. Learn. Mem. 2011, 96, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Mehta, N.D.; Haroon, E.; Xu, X.; Woolwine, B.J.; Li, Z.; Felger, J.C. Inflammation negatively correlates with amygdala-ventromedial prefrontal functional connectivity in association with anxiety in patients with depression: Preliminary results. Brain Behav. Immun. 2018, 73, 725–730. [Google Scholar] [CrossRef]
- Smith, K.J.; Au, B.; Ollis, L.; Schmitz, N. The association between C-reactive protein, interleukin-6 and depression among older adults in the community: A systematic review and meta-analysis. Exp. Gerontol. 2018, 102, 109–132. [Google Scholar] [CrossRef]
- Jha, M.K.; Minhajuddin, A.; Gadad, B.S.; Greer, T.; Grannemann, B.; Soyombo, A.; Mayes, T.L.; Rush, A.J.; Trivedi, M.H. Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology 2017, 78, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.R.; Cavanagh, J.; de Boer, P.; de Boer, P.; Mondelli, V.; Jones, D.N.C.; Drevets, W.C.; Cowen, P.J.; Harrison, N.A.; Pointon, L.; et al. Treatment-resistant depression and peripheral C-reactive protein. Br. J. Psychiatry 2019, 214, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Variables | n = 43 |
|---|---|
| Age, years (SD) | 44.7 (13.2) |
| Gender, female: male (%) | 6:37 (14.0:86.0) |
| Allergy status (%) | 25 (58.1) |
| Smoking (%) | 11 (25.6) |
| Serum hs-CRP, mg/L (SD) | 1.41 (1.07) |
| Preoperative ENS6Q, score (SD) | 15.7 (5.2) |
| Preoperative SNOT-25, score (SD) | 69.3 (21.2) |
| Preoperative BDI-II, score (SD) | 18.9 (14.7) |
| (1) normal (0–13) (%) | 18 (41.9) |
| (2) mild degree (14–19) (%) | 7 (16.3) |
| (3) moderate degree (20–28) (%) | 5 (11.6) |
| (4) severe degree (29–63) (%) | 13 (30.2) |
| Preoperative BAI, score (SD) | 19.7 (12.8) |
| (1) normal (0–7) (%) | 8 (18.6) |
| (2) mild degree (8–15) (%) | 10 (23.3) |
| (3) moderate degree (16–25) (%) | 11 (25.6) |
| (4) severe degree (26–63) (%) | 14 (32.6) |
| Variables | p-Value 1 |
|---|---|
| Demographic factor: | |
| Age | 0.537 |
| Gender | 1.000 |
| Allergy | 0.460 |
| Smoking | 0.889 |
| Pre-op status: | |
| ENS6Q | 0.704 |
| SNOT-25 | 0.099 |
| Depression (BDI-II > 13) | 0.571 |
| Anxiety (BAI > 9) | 0.755 |
| Post-op 12-month status: | |
| ENS6Q | 0.655 |
| SNOT-25 | 0.710 |
| Depression (BDI-II > 13) | 0.039 * |
| Anxiety (BAI > 9) | 0.081 |
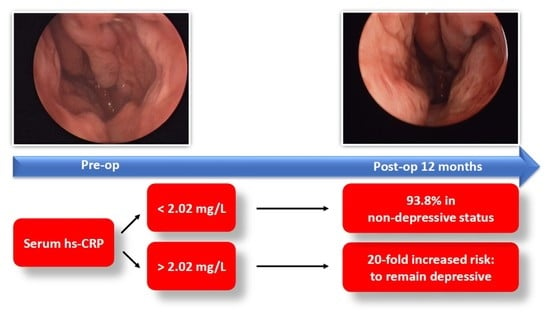
| hs-CRP Level | Non-Depression (BDI ≤ 13, n = 36) | Depression (BDI > 13, n = 7) | |
|---|---|---|---|
| ≤2.02 mg/L (n = 32) | 30 | 2 | PPV: 30/32 = 93.8% |
| >2.02 mg/L (n =11) | 6 | 5 | NPV: 5/11 = 45.4% |
| Sensitivity: 30/36 = 83.3% | Specificity: 5/7 = 71.4% |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Estimate | (95% CI) | p-Value 1 | Estimate | (95% CI) | p-Value 1 | |
| Age | 1.1 | (1.0–1.1) | <0.001 * | 0.9 | (0.8–1.0) | 0.044 * |
| Gender | - | 0.978 | - | - | ||
| Allergy | 102.3 | (11.7–893.4) | <0.001 * | - | 0.487 | |
| Smoking | 31.4 | (6.0–164.0) | <0.001 * | - | 0.092 | |
| hs-CRP > 2.02 mg/L | 140.4 | (22.6–873.4) | <0.001 * | 19.9 | (2.2–182.7) | 0.008 * |
| Pre-op ENS6Q | 1.2 | (1.1–1.4) | <0.001 * | - | 0.786 | |
| Pre-op SNOT-25 | 1.1 | (1.0–1.1) | <0.001 * | - | 0.284 | |
| Pre-op BDI-II | 1.1 | (1.1–1.2) | <0.001 * | - | 0.285 | |
| Pre-op BAI | 1.2 | (1.1–1.2) | <0.001 * | - | 0.164 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, C.-H.; Chen, H.-C.; Huang, C.-C.; Chang, P.-H.; Lee, T.-J. Serum High-Sensitivity C-Reactive Protein Is Associated with Postoperative Psychiatric Status in Patients with Empty Nose Syndrome. Diagnostics 2021, 11, 2388. https://doi.org/10.3390/diagnostics11122388
Fu C-H, Chen H-C, Huang C-C, Chang P-H, Lee T-J. Serum High-Sensitivity C-Reactive Protein Is Associated with Postoperative Psychiatric Status in Patients with Empty Nose Syndrome. Diagnostics. 2021; 11(12):2388. https://doi.org/10.3390/diagnostics11122388
Chicago/Turabian StyleFu, Chia-Hsiang, Hung-Chin Chen, Chi-Che Huang, Po-Hung Chang, and Ta-Jen Lee. 2021. "Serum High-Sensitivity C-Reactive Protein Is Associated with Postoperative Psychiatric Status in Patients with Empty Nose Syndrome" Diagnostics 11, no. 12: 2388. https://doi.org/10.3390/diagnostics11122388