Development and Clinical Validation of the LymphMonitor Technology to Quantitatively Assess Lymphatic Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Stability Tests
2.2. In Vivo Clearance in Animals
2.3. Human Study
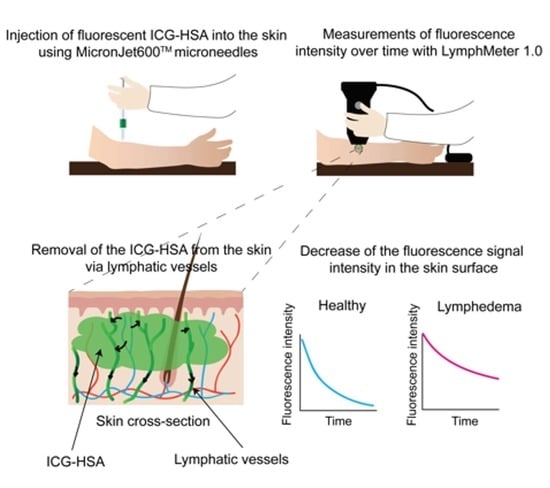
2.3.1. Study Design and Endpoints
2.3.2. Participants
2.3.3. Visits and Intervention
Screening Visit
Investigational Visit
2.3.4. Preparation of ICG-HSA
2.3.5. LymphMeter and LymphData Software
2.3.6. Data and Statistical Analysis of the Clinical Study
3. Results and Discussion
3.1. ICG-HSA Can be Used to Quantitatively Assess Lymphatic Function in Mice
3.2. In Vivo Validation of ICG-HSA in Pigs Using the LymphMeter Device
3.3. Human Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cueni, L.N.; Detmar, M. The lymphatic system in health and disease. Lymphat. Res. Biol. 2008, 6, 109–122. [Google Scholar] [CrossRef]
- Alitalo, K. The lymphatic vasculature in disease. Nat. Med. 2011, 17, 1371–1380. [Google Scholar] [CrossRef]
- Dayan, J.H.; Ly, C.L.; Kataru, R.P.; Mehrara, B.J. Lymphedema: Pathogenesis and novel therapies. Annu. Rev. Med. 2018, 69, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Bilancini, S.; Lucchi, M.; Tucci, S.; Eleuteri, P. Functional lymphatic alterations in patients suffering from lipedema. Angiology 1995, 46, 333–339. [Google Scholar] [CrossRef]
- Felmerer, G.; Stylianaki, A.; Hagerling, R.; Wang, A.; Strobel, P.; Hollmen, M.; Lindenblatt, N.; Gousopoulos, E. Adipose tissue hypertrophy, an aberrant biochemical profile and distinct gene expression in lipedema. J. Surg. Res. 2020, 253, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Felmerer, G.; Stylianaki, A.; Hollmen, M.; Strobel, P.; Stepniewski, A.; Wang, A.; Frueh, F.S.; Kim, B.S.; Giovanoli, P.; Lindenblatt, N.; et al. Increased levels of vegf-c and macrophage infiltration in lipedema patients without changes in lymphatic vascular morphology. Sci. Rep. 2020, 10, 10947. [Google Scholar] [CrossRef]
- Mortimer, P.S. Evaluation of lymphatic function: Abnormal lymph drainage in venous disease. Int. J. Angiol. 1995, 14, 32–35. [Google Scholar]
- Guo, R.; Zhou, Q.; Proulx, S.T.; Wood, R.; Ji, R.C.; Ritchlin, C.T.; Pytowski, B.; Zhu, Z.; Wang, Y.J.; Schwarz, E.M.; et al. Inhibition of lymphangiogenesis and lymphatic drainage via vascular endothelial growth factor receptor 3 blockade increases the severity of inflammation in a mouse model of chronic inflammatory arthritis. Arthritis Rheum. 2009, 60, 2666–2676. [Google Scholar] [CrossRef]
- Huggenberger, R.; Ullmann, S.; Proulx, S.T.; Pytowski, B.; Alitalo, K.; Detmar, M. Stimulation of lymphangiogenesis via vegfr-3 inhibits chronic skin inflammation. J. Exp. Med. 2010, 207, 2255–2269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurisic, G.; Sundberg, J.P.; Detmar, M. Blockade of vegf receptor-3 aggravates inflammatory bowel disease and lymphatic vessel enlargement. Inflamm. Bowel Dis. 2013, 19, 1983–1989. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Qu, H.L.; Wu, Q.; Song, Y.H. Lymphedema in survivors of breast cancer. Oncol. Lett. 2020, 19, 2085–2096. [Google Scholar]
- Cormier, J.N.; Askew, R.L.; Mungovan, K.S.; Xing, Y.; Ross, M.I.; Armer, J.M. Lymphedema beyond breast cancer: A systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer 2010, 116, 5138–5149. [Google Scholar] [CrossRef]
- Rockson, S.G. Chapter 58—diseases of the lymphatic circulation. In Vascular Medicine: A Companion to Braunwald’s Heart Disease, 2nd ed.; Creager, M.A., Beckman, J.A., Loscalzo, J., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2013; pp. 697–708. [Google Scholar]
- Taylor, M.J.; Hoerauf, A.; Bockarie, M. Lymphatic filariasis and onchocerciasis. Lancet 2010, 376, 1175–1185. [Google Scholar] [CrossRef]
- Box, R.C.; Reul-Hirche, H.M.; Bullock-Saxton, J.E.; Furnival, C.M. Physiotherapy after breast cancer surgery: Results of a randomised controlled study to minimise lymphoedema. Breast Cancer Res. Treat. 2002, 75, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Stout, N.L.; Pfalzer, L.A.; Levy, E.; McGarvey, C.; Springer, B.; Gerber, L.H.; Soballe, P. Segmental limb volume change as a predictor of the onset of lymphedema in women with early breast cancer. PmR 2011, 3, 1098–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, C.; Arthur, D.W.; Wazer, D.; Khan, A.; Ridner, S.; Vicini, F. The impact of early detection and intervention of breast cancer-related lymphedema: A systematic review. Cancer Med. 2016, 5, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, P.S.; Simmonds, R.; Rezvani, M.; Robbins, M.; Hopewell, J.W.; Ryan, T.J. The measurement of skin lymph flow by isotope clearance-reliability, reproducibility, injection dynamics, and the effect of massage. J. Investig. Dermatol. 1990, 95, 677–682. [Google Scholar] [CrossRef] [Green Version]
- Modi, S.; Stanton, A.W.; Mortimer, P.S.; Levick, J.R. Clinical assessment of human lymph flow using removal rate constants of interstitial macromolecules: A critical review of lymphoscintigraphy. Lymphat. Res. Biol. 2007, 5, 183–202. [Google Scholar] [CrossRef]
- Polomska, A.K.; Proulx, S.T. Imaging technology of the lymphatic system. Adv. Drug Deliv. Rev. 2020, 170, 294–311. [Google Scholar] [CrossRef]
- Yamamoto, T.; Narushima, M.; Yoshimatsu, H.; Yamamoto, N.; Kikuchi, K.; Todokoro, T.; Iida, T.; Koshima, I. Dynamic indocyanine green (icg) lymphography for breast cancer-related arm lymphedema. Ann. Plast. Surg. 2014, 73, 706–709. [Google Scholar] [CrossRef]
- Yamamoto, T.; Narushima, M.; Yoshimatsu, H.; Yamamoto, N.; Oka, A.; Seki, Y.; Todokoro, T.; Iida, T.; Koshima, I. Indocyanine green velocity: Lymph transportation capacity deterioration with progression of lymphedema. Ann. Plast. Surg. 2013, 71, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Unno, N.; Nishiyama, M.; Suzuki, M.; Yamamoto, N.; Inuzuka, K.; Sagara, D.; Tanaka, H.; Konno, H. Quantitative lymph imaging for assessment of lymph function using indocyanine green fluorescence lymphography. Eur. J. Vasc. Endovasc. Surg. 2008, 36, 230–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unno, N.; Nishiyama, M.; Suzuki, M.; Tanaka, H.; Yamamoto, N.; Sagara, D.; Mano, Y.; Konno, H. A novel method of measuring human lymphatic pumping using indocyanine green fluorescence lymphography. J. Vasc. Surg. 2010, 52, 946–952. [Google Scholar] [CrossRef] [Green Version]
- Unno, N.; Tanaka, H.; Suzuki, M.; Yamamoto, N.; Mano, Y.; Sano, M.; Saito, T.; Konno, H. Influence of age and gender on human lymphatic pumping pressure in the leg. Lymphology 2011, 44, 113–120. [Google Scholar]
- Saito, T.; Unno, N.; Yamamoto, N.; Inuzuka, K.; Tanaka, H.; Sano, M.; Sugisawa, R.; Katahashi, K.; Konno, H. Low lymphatic pumping pressure in the legs is associated with leg edema and lower quality of life in healthy volunteers. Lymphat. Res. Biol. 2015, 13, 154–159. [Google Scholar] [CrossRef] [Green Version]
- Polomska, A.K.; Proulx, S.T.; Brambilla, D.; Fehr, D.; Bonmarin, M.; Brandli, S.; Meboldt, M.; Steuer, C.; Vasileva, T.; Reinke, N.; et al. Minimally invasive method for the point-of-care quantification of lymphatic vessel function. J. Clin. Investig. Insight 2019, 4, e126515. [Google Scholar] [CrossRef] [Green Version]
- Karlsen, T.V.; McCormack, E.; Mujic, M.; Tenstad, O.; Wiig, H. Minimally invasive quantification of lymph flow in mice and rats by imaging depot clearance of near-infrared albumin. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H391–H401. [Google Scholar] [CrossRef] [Green Version]
- Karaman, S.; Buschle, D.; Luciani, P.; Leroux, J.-C.; Detmar, M.; Proulx, S.T. Decline of lymphatic vessel density and function in murine skin during aging. Angiogenesis 2015, 18, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Babity, S.; Polomska, A.K.; Couture, F.; Bonmarin, M.; Fehr, D.; Detmar, M.; Brambilla, D. Rational design of a fluorescent microneedle tattoo for minimally invasive monitoring of lymphatic function. J. Control. Release 2020, 327, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, D.; Proulx, S.T.; Marschalkova, P.; Detmar, M.; Leroux, J.C. Microneedles for the noninvasive structural and functional assessment of dermal lymphatic vessels. Small 2016, 12, 1053–1061. [Google Scholar] [CrossRef]
- Proulx, S.T.; Ma, Q.; Andina, D.; Leroux, J.-C.; Detmar, M. Quantitative measurement of lymphatic function in mice by noninvasive near-infrared imaging of a peripheral vein. J. Clin. Investig. Insight 2017, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Proulx, S.T.; Luciani, P.; Christiansen, A.; Karaman, S.; Blum, K.S.; Rinderknecht, M.; Leroux, J.C.; Detmar, M. Use of a peg-conjugated bright near-infrared dye for functional imaging of rerouting of tumor lymphatic drainage after sentinel lymph node metastasis. Biomaterials 2013, 34, 5128–5137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachmann, S.B.; Detmar, M.; Proulx, S.T. Visualization and measurement of lymphatic function in vivo. Methods Mol. Biol. 2018, 1846, 197–211. [Google Scholar] [PubMed]
- Makinen, T.; Jussila, L.; Veikkola, T.; Karpanen, T.; Kettunen, M.I.; Pulkkanen, K.J.; Kauppinen, R.; Jackson, D.G.; Kubo, H.; Nishikawa, S.; et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble vegf receptor-3. Nat. Med. 2001, 7, 199–205. [Google Scholar] [CrossRef]
- Levin, Y.; Kochba, E.; Hung, I.; Kenney, R. Intradermal vaccination using the novel microneedle device micronjet600: Past, present, and future. Hum. Vaccines Immunother. 2015, 11, 991–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema. Consensus document of the international society of lymphology. Lymphology 2003, 36, 84–91. [Google Scholar]
- Brorson, H.; Hoijer, P. Standardised measurements used to order compression garments can be used to calculate arm volumes to evaluate lymphoedema treatment. J. Plast. Surg. Hand Surg. 2012, 46, 410–415. [Google Scholar] [CrossRef] [Green Version]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J., Jr.; Frazier, K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef]
- Meyer, W.; Schwarz, R.; Neurand, K. The skin of domestic mammals as a model for the human skin, with special reference to the domestic pig. Curr. Probl. Derm. 1978, 7, 39–52. [Google Scholar]
- Ito, R.; Suami, H. Lymphatic territories (lymphosomes) in swine: An animal model for future lymphatic research. Plast. Reconstr. Surg. 2015, 136, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Modi, S.; Stanton, A.W.; Svensson, W.E.; Peters, A.M.; Mortimer, P.S.; Levick, J.R. Human lymphatic pumping measured in healthy and lymphoedematous arms by lymphatic congestion lymphoscintigraphy. J. Physiol. 2007, 583, 271–285. [Google Scholar] [CrossRef]
- Modi, S.; Stanton, A.W.B.; Mellor, R.H.; Michael Peters, A.; Rodney Levick, J.; Mortimer, P.S. Regional distribution of epifascial swelling and epifascial lymph drainage rate constants in breast cancer-related lymphedema. Lymphat. Res. Biol. 2005, 3, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Emmett, A.J.; Barron, J.N.; Veall, N. The use of i-131 albumin tissue clearance measurements and other physiological tests for the clinical assessment of patients with lymphoedema. Br. J. Plast. Surg. 1967, 20, 1–15. [Google Scholar] [CrossRef]
- Fernandez, M.J.; Davies, W.T.; Owen, G.M.; Tyler, A. Lymphatic flow in humans as indicated by the clearance of 125i-labeled albumin from the subcutaneous tissue of the leg. J. Surg. Res. 1983, 35, 101–104. [Google Scholar] [CrossRef]
- Staberg, B.; Klemp, P.; Aasted, M.; Worm, A.M.; Lund, P. Lymphatic albumin clearance from psoriatic skin. J. Am. Acad. Dermatol. 1983, 9, 857–861. [Google Scholar] [CrossRef]
- Pain, S.J.; Purushotham, A.D.; Barber, R.W.; Ballinger, J.R.; Solanki, C.K.; Mortimer, P.S.; Peters, A.M. Variation in lymphatic function may predispose to development of breast cancer-related lymphoedema. Ejso-Eur. J. Surg. Onc. 2004, 30, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Stanton, A.W.; Mellor, R.H.; Cook, G.J.; Svensson, W.E.; Peters, A.M.; Levick, J.R.; Mortimer, P.S. Impairment of lymph drainage in subfascial compartment of forearm in breast cancer-related lymphedema. Lymphat. Res. Biol. 2003, 1, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Stanton, A.W.B.; Svensson, W.E.; Mellor, R.H.; Peters, A.M.; Levick, J.R.; Mortimer, P.S. Differences in lymph drainage between swollen and non-swollen regions in arms with breast-cancer-related lymphoedema. Clin. Sci. 2001, 101, 131–140. [Google Scholar] [CrossRef]
- Hollander, W.; Reilly, P.; Burrows, B.A. Lymphatic flow in human subjects as indicated by disappearance of i131-labeled albumin from subcutaneous tissue. J. Clin. Investig. 1961, 40, 222. [Google Scholar] [CrossRef]
- Ellis, J.P.; Marks, R.; Perry, B.J. Lymphatic function: The disappearance rate of 131-i albumin from the dermis. Br. J. Dermatol. 1970, 82, 593–599. [Google Scholar] [CrossRef]
- O’Mahony, S.; Solanki, C.K.; Barber, R.W.; Mortimer, P.S.; Purushotham, A.D.; Peters, A.M. Imaging of lymphatic vessels in breast cancer-related lymphedema: Intradermal versus subcutaneous injection of 99mtc-immunoglobulin. Am. J. Roentgenol. 2006, 186, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Lane, K.; Worsley, D.; McKenzie, D. Lymphoscintigraphy to evaluate the effects of upper body dynamic exercise on radiopharmaceutical clearance from the hands of healthy females. Faseb J. 2005, 19, A165. [Google Scholar] [CrossRef] [PubMed]




| Inclusion Criteria |
|---|
|
| Exclusion Criteria |
|
| Patient | Age | Gender | Affected Limb | LEV (cm3) | HEV (cm3) | Etiology | Clin. Stage | BMI (kg/m2) | FD (M/Y) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 | F | right leg | 23,618 | 19,212 | injury | II | 40.1 | August 2019 |
| 2 | 63 | F | right leg | 8670 | 6613 | ovary cancer | II | 36.3 | November 2018 |
| 3 | 68 | F | left arm | 3672 | 2869 | breast cancer | II | 33.5 | July 2017 |
| 4 | 64 | F | left leg | 11,505 | 7711 | injury | II | 22 | January 2004 |
| 5 | 40 | F | left leg | 10,009 | 9333 | melanoma | II | 23.8 | October 2017 |
| 6 | 61 | M | right arm | 3158 | 2852 | infection | II | 23.5 | 2008 |
| 7 | 55 | M | right arm | 3062 | 2742 | melanoma | II | 30.3 | February 2017 |
| 8 | 49 | F | right leg | 9565 | 7735 | cervical cancer | II | 21 | 2018 |
| 9 | 48 | F | left arm | 3049 | 2765 | breast cancer | II | 29.1 | January 2013 |
| 10 | 48 | M | right leg | 10,330 | 8316 | melanoma | II | 24.5 | March 1999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polomska, A.; Gousopoulos, E.; Fehr, D.; Bachmann, A.; Bonmarin, M.; Detmar, M.; Lindenblatt, N. Development and Clinical Validation of the LymphMonitor Technology to Quantitatively Assess Lymphatic Function. Diagnostics 2021, 11, 1873. https://doi.org/10.3390/diagnostics11101873
Polomska A, Gousopoulos E, Fehr D, Bachmann A, Bonmarin M, Detmar M, Lindenblatt N. Development and Clinical Validation of the LymphMonitor Technology to Quantitatively Assess Lymphatic Function. Diagnostics. 2021; 11(10):1873. https://doi.org/10.3390/diagnostics11101873
Chicago/Turabian StylePolomska, Anna, Epameinondas Gousopoulos, Daniel Fehr, Andreas Bachmann, Mathias Bonmarin, Michael Detmar, and Nicole Lindenblatt. 2021. "Development and Clinical Validation of the LymphMonitor Technology to Quantitatively Assess Lymphatic Function" Diagnostics 11, no. 10: 1873. https://doi.org/10.3390/diagnostics11101873