Multidetector Computed Tomography with Dedicated Protocol for Breast Cancer Locoregional Staging: Feasibility Study
Abstract
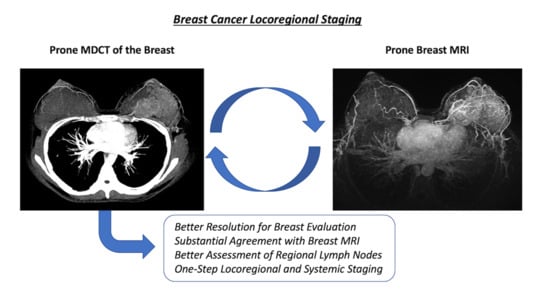
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Histopathologic Analysis
2.3. Imaging Acquisition
- Axial T1 gradient-echo phase, three-dimensional (3D) imaging (TR/TE, 545/9.7 ms; 3-mm-thick slices; FOV, 340 mm).
- Fat-saturated short tau inversion recovery (STIR) sequence in the sagittal plane of both breasts (TR/TE, 3200/63 ms; 3-mm-thick slices; FOV, 210 mm).
- Axial diffusion-weighted images (DWI) using spin-echo, single-shot echo planar imaging sequence (TR/TE, 10630/57 ms; 2.5-mm-thick slices; FOV, 350 mm; b-values: 0 and 750 s/mm2).
- Dynamic contrast enhancement (DCE): Five gradient-echo phases in T1, 3D, and in the axial plane using fat suppression for dynamic examination (TR/TE, 3.23/1.6 ms; 0.9-mm-thick slices; FOV, 360 mm), one pre-contrast and four post-contrast with a temporal resolution of 60–90 s.
- Sagittal T1-weighted, 3D gradient-echo pulse sequence with fat signal suppression (TR/TE, 4.58/2.28 ms; 1-mm-thick slices; FOV, 220 mm).
2.4. Image Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giuliano, A.E.; Connolly, J.L.; Edge, S.B.; Mittendorf, E.A.; Rugo, H.S.; Solin, L.J.; Weaver, D.L.; Winchester, D.J.; Hortobagyi, G.N. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA. Cancer J. Clin. 2017, 67, 290–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.C.; Jain, P.A.; Jethwa, S.C.; Tripathy, D.; Yamashita, M.W. Radiologist’s role in breast cancer staging: Providing key information for clinicians. Radiographics 2014, 34, 330–342. [Google Scholar] [CrossRef] [Green Version]
- Kuhl, C.K.; Lehman, C.; Bedrosian, I. Imaging in Locoregional Management of Breast Cancer. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.M.; Hayward, J.H.; Joe, B.N. Role of MR Imaging for the Locoregional Staging of Breast Cancer. Magn. Reson. Imaging Clin. N. Am. 2018, 26, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Plana, M.N.; Carreira, C.; Muriel, A.; Chiva, M.; Abraira, V.; Emparanza, J.I.; Bonfill, X.; Zamora, J. Magnetic resonance imaging in the preoperative assessment of patients with primary breast cancer: Systematic review of diagnostic accuracy and meta-analysis. Eur. Radiol. 2012, 22, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, L.R.; Duarte, C.S.; Rosa, D.D.; Edelweiss, M.I.; Edelweiss, M.; Silva, F.R.; Winnnikow, E.P.; Simões Pires, P.D.; Rosa, M.I. Accuracy of magnetic resonance in suspicious breast lesions: A systematic quantitative review and meta-analysis. Breast Cancer Res. Treat. 2011, 126, 273–285. [Google Scholar] [CrossRef]
- Mann, R.M.; Cho, N.; Moy, L. Breast MRI: State of the Art. Radiology 2019, 292, 520–536. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Gradishar, W.J.; Anderson, B.O.; Abraham, J.; Aft, R.; Allison, K.H.; Blair, S.L.; Burstein, H.J.; Dang, C.; Elias, A.D.; et al. Breast Cancer, Version 3.2018. J. Natl. Compr. Cancer Netw. 2019, 17, 118–126. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, A.M.; Karellas, A.; Vedantham, S.; Kawakyu-O’Connor, D.T. Newer Technologies in Breast Cancer Imaging: Dedicated Cone-Beam Breast Computed Tomography. Semin. Ultrasound CT MRI 2018, 39, 106–113. [Google Scholar] [CrossRef]
- Taba, S.T.; Gureyev, T.E.; Alakhras, M.; Lewis, S.; Lockie, D.; Brennan, P.C. X-Ray Phase-Contrast Technology in Breast Imaging: Principles, Options, and Clinical Application. Am. J. Roentgenol. 2018, 211, 133–145. [Google Scholar] [CrossRef]
- Doihara, H.; Fujita, T.; Takabatake, D.; Takahashi, H.; Ogasawara, Y.; Shimizu, N. Clinical Significance of Multidetector-Row Computed Tomography in Breast Surgery. Breast J. 2006, 12, S204–S209. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Sano, T.; Watai, R.; Ashikaga, R.; Ueda, K.; Watatani, M.; Nishimura, Y. Dynamic Multidetector CT of Breast Tumors: Diagnostic Features and Comparison with Conventional Techniques. Am. J. Roentgenol. 2003, 181, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Tamaki, Y.; Hamada, S.; Yamamoto, S.; Sato, Y.; Tamura, S.; Kim, S.J.; Tanji, Y.; Miyoshi, Y.; Taguchi, T.; et al. Usefulness of three-dimensional multidetector-row CT images for preoperative evaluation of tumor extension in primary breast cancer patients. Breast Cancer Res. Treat. 2005, 89, 119–125. [Google Scholar] [CrossRef]
- Kimijima, I.; Yoshida, K.; Tamura, R.; Moriya, T. Effectiveness of multi-detector row computed tomography in detection of the presence and extent of ductal carcinoma in situ. Breast Cancer 2013, 20, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.P.; Hsu, H.H.; Ko, K.H.; Chu, C.M.; Chou, Y.C.; Chang, W.C.; Chang, T.H. Differentiation of Malignant and Benign Incidental Breast Lesions Detected by Chest Multidetector-Row Computed Tomography: Added Value of Quantitative Enhancement Analysis. PLoS ONE 2016, 11, e0154569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrone, A.; Lo Mele, L.; Sassi, S.; Marini, M.; Testaverde, L.; Izzo, L.; Marini, M. MDCT of the Breast. Am. J. Roentgenol. 2008, 190, 1644–1651. [Google Scholar] [CrossRef]
- Shimauchi, A.; Yamada, T.; Sato, A.; Takase, K.; Usami, S.; Ishida, T.; Moriya, T.; Takahashi, S. Comparison of MDCT and MRI for evaluating the intraductal component of breast cancer. AJR. Am. J. Roentgenol. 2006, 187, 322–329. [Google Scholar] [CrossRef]
- Nakahara, H.; Namba, K.; Wakamatsu, H.; Watanabe, R.; Furusawa, H.; Shirouzu, M.; Matsu, T.; Tanaka, C.; Akiyama, F.; Ifuku, H.; et al. Extension of breast cancer: Comparison of CT and MRI. Radiat. Med. 2002, 20, 17–23. [Google Scholar]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Wolff, A.C.; McShane, L.M.; Hammond, M.E.H.; Allison, K.H.; Fitzgibbons, P.; Press, M.F.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Hanna, W.; et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch. Pathol. Lab. Med. 2018, 142, 1364–1382. [Google Scholar] [CrossRef] [Green Version]
- Kuroki-suzuki, S.; Kuroki, Y.; Ishikawa, T. Diagnosis of breast cancer with multidetector computed tomography: Analysis of optimal delay time after contrast media injection. J. Clin. Imaging 2010, 34, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.A.; Comstock, C.E.; Lee, C.H. ACR BI-RADS: Breast Imaging Reporting and Data System, 5th ed.; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volterrani, L.; Gentili, F.; Fausto, A.; Pelini, V.; Sardanelli, F.; Mazzei, M.A.; Volterrani, L.; Gentili, F.; Fausto, A. Dual-Energy CT for Locoregional Staging of Breast Cancer: Preliminary Results. Am. J. Roentgenol. 2020, 214, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.A.; Avendano, D.; Zapata, P.; Riedl, C.C.; Pinker, K. Lymph Node Imaging in Patients with Primary Breast Cancer: Concurrent Diagnostic Tools. Oncologist 2020, 25. [Google Scholar] [CrossRef] [Green Version]
- Nasu, Y.; Shikishima, H.; Miyasaka, Y.; Nakakubo, Y.; Ichinokawa, K.; Kaneko, T. A study of the assessment of axillary lymph nodes before surgery for breast cancer using multidetector-row computed tomography. Surg. Today 2010, 40, 1023–1026. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Doihara, H.; Shiraiwa, M.; Ishihara, S. Multidetector-row computed tomography for the preoperative evaluation of axillary nodal status in patients with breast cancer. Surg. Today 2008, 38, 104–108. [Google Scholar] [CrossRef]
- Chen, C.F.; Zhang, Y.L.; Cai, Z.L.; Sun, S.M.; Lu, X.F.; Lin, H.Y.; Liang, W.Q.; Yuan, M.H.; Zeng, D. Predictive value of preoperative multidetector-row computed tomography for axillary lymph nodes metastasis in patients with breast cancer. Front. Oncol. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Cheung, Y.C.; Chen, S.C.; Hsieh, I.C.; Lo, Y.F.; Tsai, H.P.; Hsueh, S.; Yen, T.C. Multidetector computed tomography assessment on tumor size and nodal status in patients with locally advanced breast cancer before and after neoadjuvant chemotherapy. Eur. J. Surg. Oncol. 2006, 32, 1186–1190. [Google Scholar] [CrossRef]
- Rominger, M.; Berg, D.; Frauenfelder, T.; Ramaswamy, A.; Timmesfeld, N. Which Factors Influence MRI-pathology Concordance of Tumour Size Measurements in Breast Cancer? Eur. Radiol. 2016, 26, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Haraldsdóttir, K.H.; Jónsson, Þ.; Halldórsdóttir, A.B.; Tranberg, K.G.; Ásgeirsson, K.S. Tumor Size of Invasive Breast Cancer on Magnetic Resonance Imaging and Conventional Imaging (Mammogram/Ultrasound): Comparison with Pathological Size and Clinical Implications. Scand. J. Surg. 2017, 106, 68–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Imaging Findings | MR | MDCT | Kappa | SE | p |
|---|---|---|---|---|---|
| Lesion Type | 26 (78.8%) | 25 (75.8%) | |||
| Mass | 5 (15.2%) | 0.756 | 0.117 | <0.001 | |
| NME | 6 (18.2%) | 3 (9.1%) | |||
| Both | 1 (3.0%) | ||||
| Tumor extension | |||||
| ≤2 cm (T1) | 8 (24.2%) | 9 (27.3%) | 0.674 | 0.111 | <0.001 |
| 2.1–5.0 cm (T2) | 14 (42.4%) | 15 (45.5%) | |||
| >5 cm (T3) | 11 (33.3%) | 9 (27.3%) | |||
| Additional benign biopsies | 5 (15.2%) | 1 (3.0%) | 0.298 | 0.234 | 0.016 |
| Multifocality | 12 (36.4%) | 13 (39.4%) | 0.678 | 0.132 | <0.001 |
| Multicentricity | 10 (30.3%) | 11 (33.3%) | 0.930 | 0.069 | <0.001 |
| Nipple invasion | 2 (6.1%) | 2 (6.1%) | 1.000 | 0.000 | <0.001 |
| Skin Invasion | 4 (12.1%) | 5 (15.2%) | 0.872 | 0.125 | <0.001 |
| Suspicious axillary lymph nodes | |||||
| Level I | 19 (57.6%) | 23 (69.7%) | 0.613 | 0.138 | <0.001 |
| Levels II and/or III | 1 (3.0%) | 8 (24.2%) | 0.178 | 0.157 | 0.073 |
| Number of suspected LN | |||||
| <3 | 13 (39.4%) | 12 (36.4%) | 0.628 | 0.110 | <0.001 |
| ≥3 | 6 (18.2%) | 11 (33.3%) |
| T Staging on Breast MRI and MDCT | T Staging on Pathology | Kappa (SE) | p | ||
|---|---|---|---|---|---|
| T1 | T2 | T3 | |||
| Breast MRI | |||||
| T1 (<2.1 cm) | 5 | 0 | 0 | 0.699 (0.185) | 0.002 |
| T2 (2.1–5.0 cm) | 1 * | 3 | 0 | ||
| T3 (>5 cm) | 0 | 1 # | 1 | ||
| MDCT | |||||
| T1 (<2.1 cm) | 5 | 0 | 0 | 0.699 (0.185) | 0.002 |
| T2 (2.1–5.0 cm) | 1 † | 3 | 0 | ||
| T3 (>5 cm) | 0 | 1 µ | 1 | ||
| Number of Suspected LN on Breast MRI and MDCT | Number of Metastatic LN on Pathology | Kappa (SE) | p | ||
|---|---|---|---|---|---|
| 0 | 1–2 | >2 | |||
| Breast MRI | |||||
| 0 | 3 | 4 | 1 | 0.165 (0.152) | 0.354 |
| 1–2 | 0 | 2 | 1 | ||
| >2 | 0 | 0 | 0 | ||
| MDCT | |||||
| 0 | 3 | 3 | 0 | 0.429 (0.214) | 0.038 |
| 1–2 | 0 | 3 | 1 | ||
| >2 | 0 | 0 | 1 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felipe, V.C.; Graziano, L.; Barbosa, P.N.V.P.; Calsavara, V.F.; Bitencourt, A.G.V. Multidetector Computed Tomography with Dedicated Protocol for Breast Cancer Locoregional Staging: Feasibility Study. Diagnostics 2020, 10, 479. https://doi.org/10.3390/diagnostics10070479
Felipe VC, Graziano L, Barbosa PNVP, Calsavara VF, Bitencourt AGV. Multidetector Computed Tomography with Dedicated Protocol for Breast Cancer Locoregional Staging: Feasibility Study. Diagnostics. 2020; 10(7):479. https://doi.org/10.3390/diagnostics10070479
Chicago/Turabian StyleFelipe, Vinicius C., Luciana Graziano, Paula N. V. P. Barbosa, Vinicius F. Calsavara, and Almir G. V. Bitencourt. 2020. "Multidetector Computed Tomography with Dedicated Protocol for Breast Cancer Locoregional Staging: Feasibility Study" Diagnostics 10, no. 7: 479. https://doi.org/10.3390/diagnostics10070479