The Value of Strain Elastography in Predicting Autoimmune Thyroiditis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Biochemical Assay
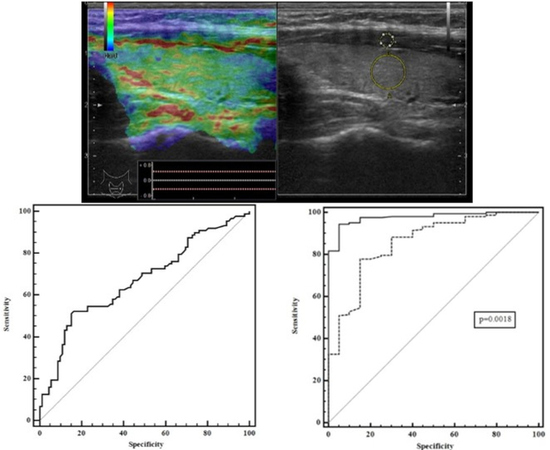
2.5. Conventional Ultrasound and Elastography Examination
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Inaba, H.; Ariyasu, H.; Takeshima, K.; Iwakura, H.; Akamizu, T. Comprehensive research on thyroid diseases associated with autoimmunity: Autoimmune thyroid diseases, thyroid diseases during immune-checkpoint inhibitors therapy, and immunoglobulin-G4-associated thyroid diseases. Endocr. J. 2019, 66, 843–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akamizu, T.; Amino, N. Hashimoto’s thyroiditis. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2017. [Google Scholar]
- Mark, P.J. Vanderpump, The epidemiology of thyroid disease. Br. Med. Bull. 2011, 99, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Biondi, B.; Cooper, D.C. The clinical significance of subclinical thyroid dysfunction. Endocr. Rev. 2008, 29, 76–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gause, W.C.; Marsh, J.A. Effect of testosterone treatments for varying periods on autoimmune development and on specific infiltrating leukocyte populations in the thyroid gland of obese strain chickens. Clin. Immunol. Immunopathol. 1986, 39, 464–478. [Google Scholar] [CrossRef]
- Vanderpump, M.P.; Tunbridge, W.M. Epidemiology and prevention of clinical and subclinical hypothyroidism. Thyroid 2002, 12, 839–847. [Google Scholar] [CrossRef]
- Tamai, H.; Ohsako, N.; Takeno, K.; Fukino, O.; Takahashi, H.; Kuma, K.; Kumagai, L.F.; Nagataki, S. Changes in thyroid function in euthyroid subjects with a family history of Graves’ disease: A follow-up study of 69 patients. J. Clin. Endocrinol. Metab. 1980, 51, 1123–1127. [Google Scholar] [CrossRef]
- Tomer, Y.; Barbesino, G.; Greenberg, D.A.; Concepcion, E.; Davies, T.F. Mapping the major susceptibility loci for familial Graves’ and Hashimoto’s diseases: Evidence for genetic heterogeneity and gene interactions. J. Clin. Endocrinol. Metab. 1999, 84, 4656–4664. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Bojunga, J. Die Bedeutung der Sonografiebei der Abklärung von Schilddrüsenerkrankungen [Ultrasound of the Thyroid]. Laryngorhinootologie 2016, 95, 87–104. [Google Scholar] [CrossRef]
- Dighe, M.; Barr, R.; Bojunga, J.; Cantisani, V.; Chammas, M.C.; Cosgrove, D.; Cui, X.W.; Dong, Y.; Fenner, F.; Radzina, M.; et al. Thyroid Ultrasound: State of the Art Part 1—Thyroid Ultrasound reporting and Diffuse Thyroid Diseases. Med. Ultrason. 2017, 19, 79–93. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Zou, D.; Cai, H.; Liu, Y. Ultrasonography in the diagnosis of Hashimoto’s thyroiditis. Front. Biosci. 2016, 21, 1006–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantisani, V.; Lodise, P.; Grazhdani, H.; Mancuso, E.; Maggini, E.; Di Rocco, G.; Catalano, C. Ultrasound elastography in the evaluation of thyroid pathology. Current status. Eur. J. Radiol. 2014, 83, 420–428. [Google Scholar] [CrossRef]
- Stoian, D.; Bogdan, T.; Craina, M.; Craciunescu, M.; Timar, R.; Schiller, A. Elastography: A new ultrasound technique in nodular thyroid pathology. Thyroid Cancer Adv. Diagn. Ther. 2016. [Google Scholar] [CrossRef] [Green Version]
- Havre, R.; Waage, J.; Gilja, O.; Ødegaard, S.; Nesje, L. Real-Time Elastography: Strain Ratio Measurements Are Influenced by the Position of the Reference Area. Ultraschall Med. Eur. J. Ultrasound 2011. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef] [PubMed]
- Zaleska-Dorobisz, U.; Kaczorowski, K. Ultrasound Elastography—Review of Techniques and its Clinical Applications. Adv. Clin. Exp. Med. 2014, 23, 645–655. [Google Scholar]
- Bojunga, J.; Herrmann, E.; Meyer, G.; Weber, S.; Zeuzem, S.; Friedrich-Rust, M. Real-Time Elastography for the Differentiation of Benign and Malignant Thyroid Nodules: A Meta-Analysis. Thyroid 2010, 20, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Stoian, D.; Timar, B.; Derban, M.; Pantea, S.; Varcus, F.; Craina, M.; Craciunescu, M. Thyroid Imaging Reporting and Data System (TI-RADS): The impact of quantitative strain elastography for better stratification of cancer risks. Med. Ultrason. 2015, 17, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Cai, J.; Wang, X. Real-time Ultrasound Elastography for Differentiation of Benign and Malignant Thyroid Nodules. J. Ultrasound Med. 2014, 33, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.; Piscaglia, F.; Bamber, J. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. 2013, 34, 238–253. [Google Scholar] [CrossRef] [Green Version]
- Kara, T.; Ateş, F.; Durmaz, M.S. Assessment of thyroid gland elasticity with shear-wave elastography in Hashimoto’s thyroiditis patients. J. Ultrasound. 2020. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Ji, Y.; Wan, Q.; Dun, G. The value of shear wave elastography in diffuse thyroid disease. Clin. Imaging 2018, 49, 187–192. [Google Scholar] [CrossRef] [PubMed]
- KuralRahatlı, F.; Turnaoğlu, H.; Kırnap, N.G. Value of shear wave elastography by virtual touch tissue imaging quantification in patients with diffuse thyroid gland pathology. Turk. J. Med. Sci. 2018, 48, 993–998. [Google Scholar] [CrossRef]
- Lin, Z.M.; Wang, Y.; Liu, C.M.; Yan, C.X.; Huang, P.T. Role of Virtual Touch Tissue Quantification in Hashimoto’s Thyroiditis. Ultrasound Med. Biol. 2018, 44, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, H.; Wang, K.; Cui, G.; Fu, F. Assessment of Diffuse Thyroid Disease by Strain Ratio in Ultrasound Elastography. Ultrasound Med. Biol. 2015, 41, 2884–2889. [Google Scholar] [CrossRef] [PubMed]
- Menzilcioglu, M.S.; Duymus, M.; Gungor, G.; Citil, S.; Sahin, T.; Boysan, S.N.; Sarica, A. The value of real-time ultrasound elastography in chronic autoimmune thyroiditis. Br. J. Radiol. 2014, 87, 20140604. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Jain, V.K.; Gupta, A.; Singh Netam, S.B.; Gupta, N. Elastography evaluation of normal thyroid gland and its comparison with diffuse non nodular enlarged thyroid gland using strain ultrasound elastography. Int. J. Res. Med. Sci. 2019, 7, 1435–1440. [Google Scholar] [CrossRef]
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef]
- Sedlackova, Z.; Herman, J.; Furst, T. Share wave elastography in diffuse thyroid disease. Biomed. Pap. 2020. [Google Scholar] [CrossRef]
- Kim, I.; Kim, E.-K.; Yoon, J.H.; Han, K.H.; Son, E.J.; Moon, H.J.; Kwak, J.Y. Diagnostic Role of Conventional Ultrasonography and ShearwaveElastography in Asymptomatic Patients with Diffuse Thyroid Disease: Initial Experience with 57 Patients. Yonsei Med. J. 2014, 55, 247. [Google Scholar] [CrossRef] [Green Version]
- Hekimoglu, K.; Yildirim, D. The role of shear wave elastography in the diagnosis of chronic autoimmune thyroiditis. Med. Ultrason. 2015, 17, 322–326. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.H.; Yoo, J.; Kim, E.-K.; Moon, H.J.; Lee, H.S.; Seo, J.Y.; Kwak, J.Y. Real-Time Elastography in the Evaluation of Diffuse Thyroid Disease: A Study Based on Elastography Histogram Parameters. Ultrasound Med. Biol. 2014, 40, 2012–2019. [Google Scholar] [CrossRef]
- Korkmaz, A.; Baki, G. Value of Real-Time and Strain Ratio Elastography in Differential Diagnosis of Graves’ Disease and Subacute and Hashimoto’s Thyroiditis. Iran. J. Radiol. 2017, 14, e22019. [Google Scholar] [CrossRef] [Green Version]
- Çekiç, B.; Parlak, A.E.; Koksel, Y.; Toslak, I.E.; Parlak, M. Real-time ultrasound elastography evaluation of the thyroid gland in adolescent patients with hashimoto thyroiditis. Biomed. Res. 2017, 28, 7534–7539. [Google Scholar]
- Fukuhara, T.; Matsuda, E.; Izawa, S.; Fujiwara, K.; Kitano, H. Utility of Shear Wave Elastography for Diagnosing Chronic Autoimmune Thyroiditis. J. Thyroid Res. 2015, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Ferraioli, G.; Filice, C.; Castera, L.; Choi, B.I.; Sporea, I.; Wilson, S.R.; Cosgrove, D.; Dietrich, C.F.; Amy, D.; Bamber, J.C.; et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: Liver. Ultrasound Med. Biol. 2015, 41, 1161–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraioli, G. Review of Liver Elastography Guidelines. J. Ultrasound Med. 2019, 38, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, E.; de Lédinghen, V.; Cassinotto, C.; Chu, W.C.; Leung, V.Y.; Ferraioli, G.; Filice, C.; Castera, L.; Vilgrain, V.; Ronot, M.; et al. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology 2018, 67, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Grosu, I.; Bob, F.; Sporea, I.; Popescu, A.; Sirli, R.; Schiller, A. Two-Dimensional Shear-Wave Elastography for Kidney Stiffness Assessment. Ultrasound Q. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gungor, O.; Guzel, F.B.; Sarica, M.A.; Gungor, G.; Ganidagli, B.; Yurttutan, N.; Gorgel, A.F.; Altunoren, O. Ultrasound Elastography Evaluations in Patient Populations With Various Kidney Diseases. Ultrasound Q. 2019, 35, 169–172. [Google Scholar] [CrossRef]
- Pearce, E.N.; Farwell, A.P.; Braverman, L.E. Thyroiditis. N. Engl. J. Med. 2003, 348, 2646–2655. [Google Scholar] [CrossRef]
- Volpé, R. The pathology of thyroiditis. Hum. Pathol. 1978, 9, 429–438. [Google Scholar] [CrossRef]
- Magri, F.; Chytiris, S.; Capelli, V.; Alessi, S.; Nalon, E.; Rotondi, M.; Chiovato, L. Shear wave elastography in the diagnosis of thyroid nodules: Feasibility in the case of coexistent chronic autoimmune Hashimoto’s thyroiditis. Clin. Endocrinol. 2011, 76, 137–141. [Google Scholar] [CrossRef] [PubMed]






| Parameter | |
|---|---|
| N | 250 |
| Age (median value and range interval) | 40.8 (18–72) |
| Gender (%): | |
| Male | N = 14/250 (5.6%) |
| Female | N = 236/250 (94.4%) |
| Thyroid volume (mean ± SD) | 12.05 ± 5.32 |
| CAT | N = 180/250 (72%) |
| Normal subjects | N = 70/250 (28%) |
| Parameter | AUROC | p-Value |
|---|---|---|
| Mean SR values cut-off vs. Max SR values cut-off | 0.87 vs. 0.8 | p = 0.116 |
| Max SR values cut-off vs. Min SR values cut-off | 0.8 vs. 0.75 | p = 0.076 |
| Mean SR values cut-off vs.Min SR values cut-off | 0.87 vs. 0.75 | p = 0.01 |
| SR Mean Values According to Age Subgroups | SR Mean Values in Normal Subjects | p-Value | ||
|---|---|---|---|---|
| 18–20 years: 3/180 | 1.44 ± 0.17 | 2/70 | 0.58 ± 0.12 | p = 0.009 |
| 21–30 years: 41/180 | 2.46 ± 1.63 | 20/70 | 1.1 ± 0.61 | p = 0.0007 |
| 31–40 years: 46/180 | 2.52 ± 1.6 | 21/70 | 1.01 ± 0.52 | p = 0.0001 |
| 41–50 years: 40/180 | 3.38 ± 3.06 | 18/70 | 1.05 ± 0.41 | p = 0.0022 |
| 51–60 years: 34/180 | 3.06 ± 1.95 | 5/70 | 1.09 ± 0.38 | p = 0.032 |
| 61–70 years: 13/180 | 3.1 ± 2.03 | 4/70 | 1.08 ± 0.73 | p = 0.07 |
| 71–80 years: 3/180 | 2.15 ± 0.07 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cepeha, C.M.; Paul, C.; Borlea, A.; Borcan, F.; Fofiu, R.; Dehelean, C.A.; Stoian, D. The Value of Strain Elastography in Predicting Autoimmune Thyroiditis. Diagnostics 2020, 10, 874. https://doi.org/10.3390/diagnostics10110874
Cepeha CM, Paul C, Borlea A, Borcan F, Fofiu R, Dehelean CA, Stoian D. The Value of Strain Elastography in Predicting Autoimmune Thyroiditis. Diagnostics. 2020; 10(11):874. https://doi.org/10.3390/diagnostics10110874
Chicago/Turabian StyleCepeha, Cristina Mihaela, Corina Paul, Andreea Borlea, Florin Borcan, Renata Fofiu, Cristina Adriana Dehelean, and Dana Stoian. 2020. "The Value of Strain Elastography in Predicting Autoimmune Thyroiditis" Diagnostics 10, no. 11: 874. https://doi.org/10.3390/diagnostics10110874