Liver Biopsy Hydroxyproline Content Is a Diagnostic for Hepatocellular Carcinoma in Murine Models of Nonalcoholic Steatohepatitis
Abstract
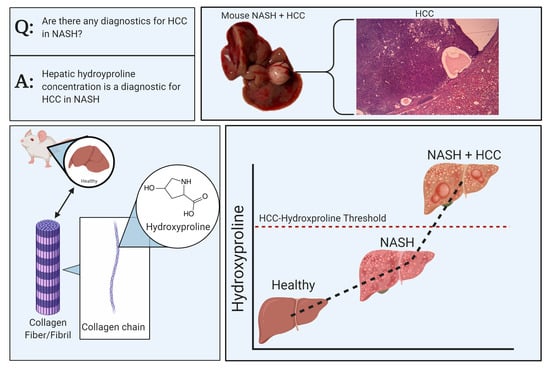
:1. Introduction
2. Materials and Methods
2.1. Animal Models
2.2. Histopathology
2.3. Liver Function Tests
2.4. Liver Hydroxyproline
2.5. Data Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Tesfay, M.; Goldkamp, W.J.; Neuschwander-Tetri, B.A. NASH: The Emerging Most Common Form of Chronic Liver Disease. Missouri Med. 2018, 115, 225–229. [Google Scholar] [PubMed]
- Sanyal, A.J.; Friedman, S.L.; McCullough, A.J.; Dimick-Santos, L. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: Findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. American Association for the Study of Liver Diseases; United States Food and Drug Administration. Hepatology 2015, 61, 1392–1405. [Google Scholar]
- Hwang, A.; Shi, C.; Zhu, E.; Naaz, F.; Zhou, P.; Rasheed, Z.; Liu, M.; Jung, L.S.; Duan, B.; Li, J.; et al. Supervised Learning Reveals Circulating Biomarker Levels Diagnostic of Hepatocellular Carcinoma in a Clinically Relevant Model of Non-alcoholic Steatohepatitis; An OAD to NASH. PLoS ONE 2018, 13, e0198937. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. We’ve hit the Iceberg – The threat of NASH-related HCC arrives. J. Hepatol. 2014, 60, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Perumpail, R.B.; Wong, R.J.; Ahmed, A.; Harrison, S.A. Hepatocellular carcinoma in the setting of non-cirrhotic nonalcoholic fatty liver disease and the metabolic syndrome: US experience. Dig. Dis. Sci. 2015, 60, 3142–3148. [Google Scholar] [CrossRef] [PubMed]
- Ertle, J.; Dechene, A.; Sowa, J.-P.; Penndorf, V.; Herzer, K.; Kaiser, G.; Schlaak, J.F.; Gerken, G.; Syn, W.-K.; Canbay, A. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int. J. Cancer 2011, 128, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Kolly, P.; Dufour, J.F. Surveillance for hepatocellular carcinoma in patients with NASH. Diagnostics 2016, 6, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cholankeril, G.; Patel, R.; Khurana, S.; Satapathy, S.K. Hepatocellular carcinoma in non-alcoholic steatohepatitis: Current knowledge and implications for management. World J. Hepatol. 2017, 9, 533–543. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, M.M.; Abecassis, L.R.; Roberts, C.B.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. Aasld guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [Green Version]
- Pons, F.; Varela, M.; Llovet, J.M. Staging systems in hepatocellular carcinoma. Hpb 2005, 7, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, R.; Lahiri, N. Tissue-and serum-associated biomarkers of hepatocellular carcinoma. Biomarkers Cancer 2016, 8, S34413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cissell, D.D.; Link, J.M.; Hu, J.C.; Athanasiou, K.A. A Modified Hydroxyproline Assay Based on Hydrochloric Acid in Ehrlich’s Solution Accurately Measures Tissue Collagen Content. Tissue Eng. Part C Methods 2017, 23, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Paka, L.; Smith, D.E.; Jung, D.; McCormack, S.; Zhou, P.; Duan, B.; Li, J.S.; Shi, J.; Hao, Y.J.; Jiang, K.; et al. Anti-steatotic and anti-fibrotic effects of the KCa3.1 channel inhibitor, Senicapoc, in non-alcoholic liver disease. World J. Gastroenterol. 2017, 23, 4181–4190. [Google Scholar] [CrossRef]
- Chavre, B.M.; Jiang, K.; St Surin, L.G.; Bissoondial, T.; Zhou, P.; Li, J.; Gadhiya, S.V.; Goldberg, I.D.; Narayan, P. Remodeling of Intrahepatic Ducts in a Model of Caroli Syndrome: Is Scar Carcinoma a Consequence of Laplace’s Law? Med. Sci. 2019, 7, 55. [Google Scholar] [CrossRef] [Green Version]
- Narayan, R.; Li, J.; Pellicano, A.J.; Goldberg, I.D. Collagen-driven remodeling of the intrahepatic duct wall in the PCK rat model of polycystic kidney disease-Caroli syndrome. Clin. Exp. HEPATOL 2020, 6, 131. [Google Scholar] [CrossRef]
- Tsuchida, T.; Lee, Y.A.; Fujiwara, N.; Ybanez, M.; Allen, B.; Martins, S.; Fiel, M.I.; Goossens, N.; Chou, H.I.; Hoshida, Y.; et al. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J. Hepatol. 2018, 69, 385–395. [Google Scholar] [CrossRef]
- Liao, K.; Pellicano, A.J.; Jiang, K.; Prakash, N.; Li, J.; Bhutkar, S.; Hu, Z.; Ali, Q.; Goldberg, I.D.; Narayan, P. Glycerol-3-phosphate Acyltransferase1 Is a Model-Agnostic Node in Nonalcoholic Fatty Liver Disease: Implications for Drug Development and Precision Medicine. ACS Omega 2020, 5, 18465–18471. [Google Scholar] [CrossRef]
- Huang, B.; Abbott, N.A.E.; Dacon, L.; McCormack, S.; Zhou, P.; Zhang, L.; Duan, B.; Li, J.; Zhang, B.; Yamin, M.; et al. Acute Injury in Natural Diet-Induced Fatty Livers—A Model for Therapy Development. Recent Pat. Biomark. 2015, 5, 101–107. [Google Scholar] [CrossRef]
- Scalera, A.; Tarantino, G. Could metabolic syndrome lead to hepatocarcinoma via non-alcoholic fatty liver disease? World J. Gastroenterol. 2014, 20, 9217–9228. [Google Scholar] [PubMed] [Green Version]
- Owada, Y.; Tamura, T.; Tanoi, T.; Ozawa, Y.; Shimizu, Y.; Hisakura, K.; Matsuzaka, T.; Shimano, H.; Nakano, N.; Sakashita, S.; et al. Novel non-alcoholic steatohepatitis model with histopathological and insulin-resistant features. Pathol. Int. 2018, 68, 12–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, L.; Gupta, D.; Abdullah, S.T. Thioacetamide potentiates high cholesterol and high fat diet induced steato-hepatitic changes in livers of C57BL/6J mice: A novel eight weeks model of fibrosing NASH. Toxicol. Lett. 2019, 304, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Yanguas, S.C.; Cogliati, B.; Willebrords, J.; Maes, M.; Colle, I.; van den Bossche, B.; de Oliviera, C.P.M.S.; Andraus, W.; Alves, V.A.; Leclercq, I.; et al. Experimental models of liver fibrosis. Arch. Toxicol. 2016, 90, 1025–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Sample | Tumor | HYP (μg/mg Liver) | HCC | False Positive |
|---|---|---|---|---|
| 1 | no | 0.17 | no | |
| 2 | no | 0.16 | no | |
| 3 | no | 0.18 | no | |
| 4 | yes | 0.45 | yes | |
| 5 | yes | 0.50 | yes | |
| 6 | no | 0.55 | yes | yes |
| 7 | yes | 0.53 | yes | |
| 8 | yes | 0.40 | yes | |
| 9 | yes | 0.19 | yes | |
| 10 | no | 0.18 | no | |
| 11 | no | 0.23 | yes | yes |
| 12 | no | 0.16 | no | |
| 13 | yes | 0.30 | yes | |
| 14 | no | 0.18 | no | |
| 15 | no | 0.18 | no |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bissoondial, T.L.; Han, Y.; Mullan, S.; Pabla, A.K.; Spahn, K.; Shi, S.; Zheng, L.; Zhou, P.; Jiang, K.; Prakash, N.; et al. Liver Biopsy Hydroxyproline Content Is a Diagnostic for Hepatocellular Carcinoma in Murine Models of Nonalcoholic Steatohepatitis. Diagnostics 2020, 10, 784. https://doi.org/10.3390/diagnostics10100784
Bissoondial TL, Han Y, Mullan S, Pabla AK, Spahn K, Shi S, Zheng L, Zhou P, Jiang K, Prakash N, et al. Liver Biopsy Hydroxyproline Content Is a Diagnostic for Hepatocellular Carcinoma in Murine Models of Nonalcoholic Steatohepatitis. Diagnostics. 2020; 10(10):784. https://doi.org/10.3390/diagnostics10100784
Chicago/Turabian StyleBissoondial, Tyler L., Yiguang Han, Stephanie Mullan, Amrit K. Pabla, Kiera Spahn, Steven Shi, Lana Zheng, Ping Zhou, Kai Jiang, Natalia Prakash, and et al. 2020. "Liver Biopsy Hydroxyproline Content Is a Diagnostic for Hepatocellular Carcinoma in Murine Models of Nonalcoholic Steatohepatitis" Diagnostics 10, no. 10: 784. https://doi.org/10.3390/diagnostics10100784